An official website of the United States government
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( Lock Locked padlock icon ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.
- Publications
- Account settings
- Advanced Search
- Journal List


Genetically modified foods: safety, risks and public concerns—a review
K r anilakumar.
- Author information
- Article notes
- Copyright and License information
Corresponding author.
Revised 2012 Nov 16; Accepted 2012 Nov 19; Issue date 2013 Dec.
Genetic modification is a special set of gene technology that alters the genetic machinery of such living organisms as animals, plants or microorganisms. Combining genes from different organisms is known as recombinant DNA technology and the resulting organism is said to be ‘Genetically modified (GM)’, ‘Genetically engineered’ or ‘Transgenic’. The principal transgenic crops grown commercially in field are herbicide and insecticide resistant soybeans, corn, cotton and canola. Other crops grown commercially and/or field-tested are sweet potato resistant to a virus that could destroy most of the African harvest, rice with increased iron and vitamins that may alleviate chronic malnutrition in Asian countries and a variety of plants that are able to survive weather extremes. There are bananas that produce human vaccines against infectious diseases such as hepatitis B, fish that mature more quickly, fruit and nut trees that yield years earlier and plants that produce new plastics with unique properties. Technologies for genetically modifying foods offer dramatic promise for meeting some areas of greatest challenge for the 21st century. Like all new technologies, they also pose some risks, both known and unknown. Controversies and public concern surrounding GM foods and crops commonly focus on human and environmental safety, labelling and consumer choice, intellectual property rights, ethics, food security, poverty reduction and environmental conservation. With this new technology on gene manipulation what are the risks of “tampering with Mother Nature”?, what effects will this have on the environment?, what are the health concerns that consumers should be aware of? and is recombinant technology really beneficial? This review will also address some major concerns about the safety, environmental and ecological risks and health hazards involved with GM foods and recombinant technology.
Keywords: Genetically modified foods, Genetically engineered foods, Transgenic foods, Food safety, Allergenic foods, Public concerns
Introduction
Scientists first discovered in 1946 that DNA can be transferred between organisms (Clive 2011 ). It is now known that there are several mechanisms for DNA transfer and that these occur in nature on a large scale, for example, it is a major mechanism for antibiotic resistance in pathogenic bacteria. The first genetically modified (GM) plant was produced in 1983, using an antibiotic-resistant tobacco plant. China was the first country to commercialize a transgenic crop in the early 1990s with the introduction of virus resistant tobacco. In 1994, the transgenic ‘Flavour Saver tomato’ was approved by the Food and Drug Administration (FDA) for marketing in the USA. The modification allowed the tomato to delay ripening after picking. In 1995, few transgenic crops received marketing approval. This include canola with modified oil composition (Calgene), Bacillus thuringiensis (Bt) corn/maize (Ciba-Geigy), cotton resistant to the herbicide bromoxynil (Calgene), Bt cotton (Monsanto), Bt potatoes (Monsanto), soybeans resistant to the herbicide glyphosate (Monsanto), virus-resistant squash (Asgrow) and additional delayed ripening tomatoes (DNAP, Zeneca/Peto, and Monsanto) (Clive 2011 ). A total of 35 approvals had been granted to commercially grow 8 transgenic crops and one flower crop of carnations with 8 different traits in 6 countries plus the EU till 1996 (Clive 1996 ). As of 2011, the USA leads a list of multiple countries in the production of GM crops. Currently, there are a number of food species in which a genetically modified version exists (Johnson 2008 ). Some of the foods that are available in the market include cotton, soybean, canola, potatoes, eggplant, strawberries, corn, tomatoes, lettuce, cantaloupe, carrots etc. GM products which are currently in the pipeline include medicines and vaccines, foods and food ingredients, feeds and fibres. Locating genes for important traits, such as those conferring insect resistance or desired nutrients-is one of the most limiting steps in the process.
Foods derived from GM crops
At present there are several GM crops used as food sources. As of now there are no GM animals approved for use as food, but a GM salmon has been proposed for FDA approval. In instances, the product is directly consumed as food, but in most of the cases, crops that have been genetically modified are sold as commodities, which are further processed into food ingredients.
Fruits and vegetables
Papaya has been developed by genetic engineering which is ring spot virus resistant and thus enhancing the productivity. This was very much in need as in the early 1990s the Hawaii’s papaya industry was facing disaster because of the deadly papaya ring spot virus. Its single-handed savior was a breed engineered to be resistant to the virus. Without it, the state’s papaya industry would have collapsed. Today 80 % of Hawaiian papaya is genetically engineered, and till now no conventional or organic method is available to control ring spot virus.
The NewLeaf™ potato, a GM food developed using naturally-occurring bacteria found in the soil known as Bacillus thuringiensis (Bt), was made to provide in-plant protection from the yield-robbing Colorado potato beetle. This was brought to market by Monsanto in the late 1990s, developed for the fast food market. This was forced to withdraw from the market in 2001as the fast food retailers did not pick it up and thereby the food processors ran into export problems. Reports say that currently no transgenic potatoes are marketed for the purpose of human consumption. However, BASF, one of the leading suppliers of plant biotechnology solutions for agriculture requested for the approval for cultivation and marketing as a food and feed for its ‘Fortuna potato’. This GM potato was made resistant to late blight by adding two resistance genes, blb1 and blb2, which was originated from the Mexican wild potato Solanum bulbocastanum . As of 2005, about 13 % of the zucchini grown in the USA is genetically modified to resist three viruses; the zucchini is also grown in Canada (Johnson 2008 ).
Vegetable oil
It is reported that there is no or a significantly small amount of protein or DNA remaining in vegetable oil extracted from the original GM crops in USA. Vegetable oil is sold to consumers as cooking oil, margarine and shortening, and is used in prepared foods. Vegetable oil is made of triglycerides extracted from plants or seeds and then refined, and may be further processed via hydrogenation to turn liquid oils into solids. The refining process removes nearly all non-triglyceride ingredients (Crevel et al. 2000 ). Cooking oil, margarine and shortening may also be made from several crops. A large percentage of Canola produced in USA is GM and is mainly used to produce vegetable oil. Canola oil is the third most widely consumed vegetable oil in the world. The genetic modifications are made for providing resistance to herbicides viz. glyphosate or glufosinate and also for improving the oil composition. After removing oil from canola seed, which is ∼43 %, the meal has been used as high quality animal feed. Canola oil is a key ingredient in many foods and is sold directly to consumers as margarine or cooking oil. The oil has many non-food uses, which includes making lipsticks.
Maize, also called corn in the USA and cornmeal, which is ground and dried maize constitute a staple food in many regions of the world. Grown since 1997 in the USA and Canada, 86 % of the USA maize crop was genetically modified in 2010 (Hamer and Scuse 2010 ) and 32 % of the worldwide maize crop was GM in 2011 (Clive 2011 ). A good amount of the total maize harvested go for livestock feed including the distillers grains. The remaining has been used for ethanol and high fructose corn syrup production, export, and also used for other sweeteners, cornstarch, alcohol, human food or drink. Corn oil is sold directly as cooking oil and to make shortening and margarine, in addition to make vitamin carriers, as a source of lecithin, as an ingredient in prepared foods like mayonnaise, sauces and soups, and also to fry potato chips and French fries. Cottonseed oil is used as a salad and cooking oil, both domestically and industrially. Nearly 93 % of the cotton crop in USA is GM.
The USA imports 10 % of its sugar from other countries, while the remaining 90 % is extracted from domestically grown sugar beet and sugarcane. Out of the domestically grown sugar crops, half of the extracted sugar is derived from sugar beet, and the other half is from sugarcane. After deregulation in 2005, glyphosate-resistant sugar beet was extensively adopted in the USA. In USA 95 % of sugar beet acres were planted with glyphosate-resistant seed (Clive 2011 ). Sugar beets that are herbicide-tolerant have been approved in Australia, Canada, Colombia, EU, Japan, Korea, Mexico, New Zealand, Philippines, Russian Federation, Singapore and USA. The food products of sugar beets are refined sugar and molasses. Pulp remaining from the refining process is used as animal feed. The sugar produced from GM sugar beets is highly refined and contains no DNA or protein—it is just sucrose, the same as sugar produced from non-GM sugar beets (Joana et al. 2010 ).
Quantification of genetically modified organisms (GMOs) in foods
Testing on GMOs in food and feed is routinely done using molecular techniques like DNA microarrays or qPCR. These tests are based on screening genetic elements like p35S, tNos, pat, or bar or event specific markers for the official GMOs like Mon810, Bt11, or GT73. The array based method combines multiplex PCR and array technology to screen samples for different potential GMO combining different approaches viz. screening elements, plant-specific markers, and event-specific markers. The qPCR is used to detect specific GMO events by usage of specific primers for screening elements or event specific markers. Controls are necessary to avoid false positive or false negative results. For example, a test for CaMV is used to avoid a false positive in the event of a virus contaminated sample.
Joana et al. ( 2010 ) reported the extraction and detection of DNA along with a complete industrial soybean oil processing chain to monitor the presence of Roundup Ready (RR) soybean. The amplification of soybean lectin gene by end-point polymerase chain reaction (PCR) was achieved in all the steps of extraction and refining processes. The amplification of RR soybean by PCR assays using event specific primers was also achieved for all the extraction and refining steps. This excluded the intermediate steps of refining viz. neutralization, washing and bleaching possibly due to sample instability. The real-time PCR assays using specific probes confirmed all the results and proved that it is possible to detect and quantify GMOs in the fully refined soybean oil.
Figure 1 gives the overall protocol for the testing of GMOs. This is based on a PCR detection system specific for 35S promoter region originating from cauliflower mosaic virus (Deisingh and Badrie 2005 ). The 35S-PCR technique permits detection of GMO contents of foods and raw materials in the range of 0.01–0.1 %. The development of quantitative detection systems such as quantitative competitive PCR (QC-PCR), real-time PCR and ELISA systems resulted in the advantage of survival of DNA in most manufacturing processes. Otherwise with ELISA, there can be protein denaturing during food processing. Inter-laboratory differences were found to be less with the QC-PCR than with quantitative PCR probably due to insufficient homogenisation of the sample. However, there are disadvantages, the major one being the amount of DNA, which could be amplified, is affected by food processing techniques and can vary up to 5-fold. Thus, results need to be normalised by using plant-specific QC-PCR system. Further, DNA, which cannot be amplified, will affect all quantitative PCR detection systems.
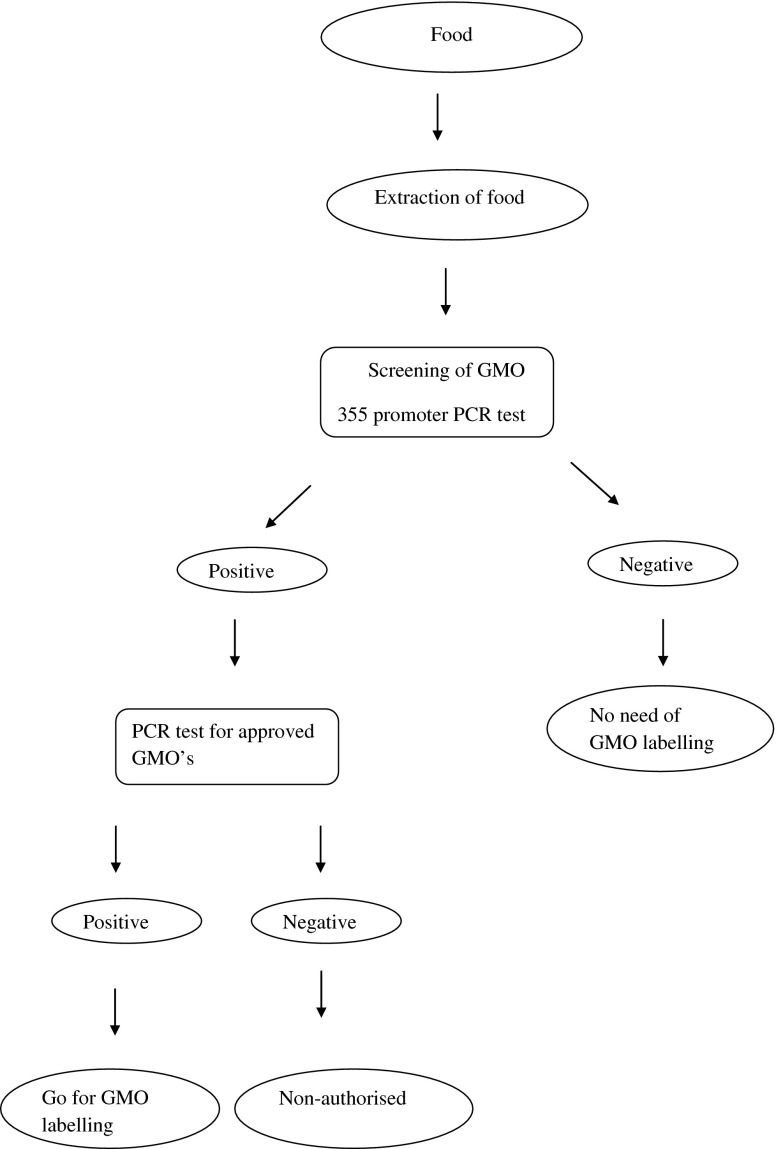
Protocol for the testing of genetically modified foods
In a recent work La Mura et al. ( 2011 ) applied QUIZ (quantization using informative zeros) to estimate the contents of RoundUp Ready™ soya and MON810 in processed food containing one or both GMs. They reported that the quantification of GM in samples can be performed without the need for certified reference materials using QUIZ. Results showed good agreement between derived values and known input of GM material and compare favourably with quantitative real-time PCR. Detection of Roundup Ready soybean by loop-mediated isothermal amplification combined with a lateral-flow dipstick has been reported recently (Xiumin et al. 2012 ).
GM foods-merits and demerits
Before we think of having GM foods it is very important to know about is advantages and disadvantages especially with respect to its safety. These foods are made by inserting genes of other species into their DNA. Though this kind of genetic modification is used both in plants and animals, it is found more commonly in the former than in the latter. Experts are working on developing foods that have the ability to alleviate certain disorders and diseases. Though researchers and the manufacturers make sure that there are various advantages of consuming these foods, a fair bit of the population is entirely against them.
GM foods are useful in controlling the occurrence of certain diseases. By modifying the DNA system of these foods, the properties causing allergies are eliminated successfully. These foods grow faster than the foods that are grown traditionally. Probably because of this, the increased productivity provides the population with more food. Moreover these foods are a boon in places which experience frequent droughts, or where the soil is incompetent for agriculture. At times, genetically engineered food crops can be grown at places with unfavourable climatic conditions too. A normal crop can grow only in specific season or under some favourable climatic conditions. Though the seeds for such foods are quite expensive, their cost of production is reported to be less than that of the traditional crops due to the natural resistance towards pests and insects. This reduces the necessity of exposing GM crops to harmful pesticides and insecticides, making these foods free from chemicals and environment friendly as well. Genetically engineered foods are reported to be high in nutrients and contain more minerals and vitamins than those found in traditionally grown foods. Other than this, these foods are known to taste better. Another reason for people opting for genetically engineered foods is that they have an increased shelf life and hence there is less fear of foods getting spoiled quickly.
The biggest threat caused by GM foods is that they can have harmful effects on the human body. It is believed that consumption of these genetically engineered foods can cause the development of diseases which are immune to antibiotics. Besides, as these foods are new inventions, not much is known about their long term effects on human beings. As the health effects are unknown, many people prefer to stay away from these foods. Manufacturers do not mention on the label that foods are developed by genetic manipulation because they think that this would affect their business, which is not a good practice. Many religious and cultural communities are against such foods because they see it as an unnatural way of producing foods. Many people are also not comfortable with the idea of transferring animal genes into plants and vice versa. Also, this cross-pollination method can cause damage to other organisms that thrive in the environment. Experts are also of the opinion that with the increase of such foods, developing countries would start depending more on industrial countries because it is likely that the food production would be controlled by them in the time to come.
Safety tests on commercial GM crops
The GM tomatoes were produced by inserting kanr genes into a tomato by an ‘antisense’ GM method (IRDC 1998 ). The results show that there were no significant alterations in total protein, vitamins and mineral contents and in toxic glycoalkaloids (Redenbaugh et al. 1992 ). Therefore, the GM and parent tomatoes were deemed to be “substantially equivalent”. In acute toxicity studies with male/female rats, which were tube-fed with homogenized GM tomatoes, toxic effects were reported to be absent. A study with a GM tomato expressing B. thuringiensis toxin CRYIA (b) was underlined by the immunocytochemical demonstration of in vitro binding of Bt toxin to the caecum/colon from humans and rhesus monkeys (Noteborn et al. 1995 ).
Two lines of Chardon LL herbicide-resistant GM maize expressing the gene of phosphinothricin acetyltransferase before and after ensiling showed significant differences in fat and carbohydrate contents compared with non-GM maize and were therefore substantially different come. Toxicity tests were only performed with the maize even though with this the unpredictable effects of the gene transfer or the vector or gene insertion could not be demonstrated or excluded. The design of these experiments was also flawed because of poor digestibility and reduction in feed conversion efficiency of GM corn. One broiler chicken feeding study with rations containing transgenic Event 176 derived Bt corn (Novartis) has been published (Brake and Vlachos 1998 ). However, the results of this trial are more relevant to commercial than academic scientific studies.
GM soybeans
To make soybeans herbicide resistant, the gene of 5-enolpyruvylshikimate-3-phosphate synthase from Agrobacterium was used. Safety tests claim the GM variety to be “substantially equivalent” to conventional soybeans (Padgette et al. 1996 ). The same was claimed for GTS (glyphosate-resistant soybeans) sprayed with this herbicide (Taylor et al. 1999 ). However, several significant differences between the GM and control lines were recorded (Padgette et al. 1996 ) and the study showed statistically significant changes in the contents of genistein (isoflavone) with significant importance for health (Lappe et al. 1999 ) and increased content in trypsin inhibitor.
Studies have been conducted on the feeding value (Hammond et al. 1996 ) and possible toxicity (Harrison et al. 1996 ) for rats, broiler chickens, catfish and dairy cows of two GM lines of glyphosate-resistant soybean (GTS). The growth, feed conversion efficiency, catfish fillet composition, broiler breast muscle and fat pad weights and milk production, rumen fermentation and digestibilities in cows were found to be similar for GTS and non-GTS. These studies had the following lacunae: (a) No individual feed intakes, body or organ weights were given and histology studies were qualitative microscopy on the pancreas, (b) The feeding value of the two GTS lines was not substantially equivalent either because the rats/catfish grew significantly better on one of the GTS lines than on the other, (c) The design of study with broiler chicken was not much convincing, (d) Milk production and performance of lactating cows also showed significant differences between cows fed GM and non-GM feeds and (e) Testing of the safety of 5-enolpyruvylshikimate-3-phosphate synthase, which renders soybeans glyphosate-resistant (Harrison et al. 1996 ), was irrelevant because in the gavage studies an E. coli recombinant and not the GTS product were used. In a separate study (Teshima et al. 2000 ), it was claimed that rats and mice which were fed 30 % toasted GTS or non-GTS in their diet had no significant differences in nutritional performance, organ weights, histopathology and production of IgE and IgG antibodies.
GM potatoes
There were no improvements in the protein content or amino acid profile of GM potatoes (Hashimoto et al. 1999a ). In a short feeding study to establish the safety of GM potatoes expressing the soybean glycinin gene, rats were daily force-fed with 2 g of GM or control potatoes/kg body weight (Hashimoto et al 1999b ). No differences in growth, feed intake, blood cell count and composition and organ weights between the groups were found. In this study, the intake of potato by animals was reported to be too low (Pusztai 2001 ).
Feeding mice with potatoes transformed with a Bacillus thuringiensis var. kurstaki Cry1 toxin gene or the toxin itself was shown to have caused villus epithelial cell hypertrophy and multinucleation, disrupted microvilli, mitochondrial degeneration, increased numbers of lysosomes and autophagic vacuoles and activation of crypt Paneth cells (Fares and El-Sayed 1998 ). The results showed CryI toxin which was stable in the mouse gut. Growing rats pair-fed on iso -proteinic and iso -caloric balanced diets containing raw or boiled non-GM potatoes and GM potatoes with the snowdrop ( Galanthus nivalis ) bulb lectin (GNA) gene (Ewen and Pusztai 1999 ) showed significant increase in the mucosal thickness of the stomach and the crypt length of the intestines of rats fed GM potatoes. Most of these effects were due to the insertion of the construct used for the transformation or the genetic transformation itself and not to GNA which had been pre-selected as a non-mitotic lectin unable to induce hyperplastic intestinal growth (Pusztai et al. 1990 ) and epithelial T lymphocyte infiltration.
The kind that expresses soybean glycinin gene (40–50 mg glycinin/g protein) was developed (Momma et al. 1999 ) and was claimed to contain 20 % more protein. However, the increased protein content was found probably due to a decrease in moisture rather than true increase in protein.
Several lines of GM cotton plants have been developed using a gene from Bacillus thuringiensis subsp. kurstaki providing increased protection against major lepidopteran pests. The lines were claimed to be “substantially equivalent” to parent lines (Berberich et al. 1996 ) in levels of macronutrients and gossypol. Cyclopropenoid fatty acids and aflatoxin levels were less than those in conventional seeds. However, because of the use of inappropriate statistics it was questionable whether the GM and non-GM lines were equivalent, particularly as environmental stresses could have unpredictable effects on anti-nutrient/toxin levels (Novak and Haslberger 2000 ).
The nutritional value of diets containing GM peas expressing bean alpha-amylase inhibitor when fed to rats for 10 days at two different doses viz. 30 % and 65 % was shown to be similar to that of parent-line peas (Pusztai et al. 1999 ). At the same time in order to establish its safety for humans a more rigorous specific risk assessment will have to be carried out with several GM lines. Nutritional/toxicological testing on laboratory animals should follow the clinical, double-blind, placebo-type tests with human volunteers.
Allergenicity studies
When the gene is from a crop of known allergenicity, it is easy to establish whether the GM food is allergenic using in vitro tests, such as RAST or immunoblotting, with sera from individuals sensitised to the original crop. This was demonstrated in GM soybeans expressing the brasil nut 2S proteins (Nordlee et al. 1996 ) or in GM potatoes expressing cod protein genes (Noteborn et al. 1995 ). It is also relatively easy to assess whether genetic engineering affected the potency of endogenous allergens (Burks and Fuchs 1995 ). Farm workers exposed to B. thuringiensis pesticide were shown to have developed skin sensitization and IgE antibodies to the Bt spore extract. With their sera it may now therefore be possible to test for the allergenic potential of GM crops expressing Bt toxin (Bernstein et al. 1999 ). It is all the more important because Bt toxin Cry1Ac has been shown to be a potent oral/nasal antigen and adjuvant (Vazquez-Padron et al. 2000 ).
The decision-tree type of indirect approach based on factors such as size and stability of the transgenically expressed protein (O’Neil et al. 1998 ) is even more unsound, particularly as its stability to gut proteolysis is assessed by an in vitro (simulated) testing (Metcalf et al. 1996 ) instead of in vivo (human/animal) testing and this is fundamentally wrong. The concept that most allergens are abundant proteins may be misleading because, for example, Gad c 1, the major allergen in codfish, is not a predominant protein (Vazquez-Padron et al. 2000 ). However, when the gene responsible for the allergenicity is known, such as the gene of the alpha-amylase/trypsin inhibitors/allergens in rice, cloning and sequencing opens the way for reducing their level by antisense RNA strategy (Nakamura and Matsuda 1996 ).
It is known that the main concerns about adverse effects of GM foods on health are the transfer of antibiotic resistance, toxicity and allergenicity. There are two issues from an allergic standpoint. These are the transfer of a known allergen that may occur from a crop into a non-allergenic target crop and the creation of a neo-allergen where de novo sensitisation occurs in the population. Patients allergic to Brazil nuts and not to soy bean then showed an IgE mediated response towards GM soy bean. Lack ( 2002 ) argued that it is possible to prevent such occurrences by doing IgE-binding studies and taking into account physico-chemical characteristics of proteins and referring to known allergen databases. The second possible scenario of de novo sensitisation does not easily lend itself to risk assessment. He reports that evidence that the technology used for the production of GM foods poses an allergic threat per se is lacking very much compared to other methodologies widely accepted in the food industry.
Risks and controversy
There are controversies around GM food on several levels, including whether food produced with it is safe, whether it should be labelled and if so how, whether agricultural biotechnology and it is needed to address world hunger now or in the future, and more specifically with respect to intellectual property and market dynamics, environmental effects of GM crops and GM crops’ role in industrial agricultural more generally.
Many problems, viz. the risks of “tampering with Mother Nature”, the health concerns that consumers should be aware of and the benefits of recombinant technology, also arise with pest-resistant and herbicide-resistant plants. The evolution of resistant pests and weeds termed superbugs and super weeds is another problem. Resistance can evolve whenever selective pressure is strong enough. If these cultivars are planted on a commercial scale, there will be strong selective pressure in that habitat, which could cause the evolution of resistant insects in a few years and nullify the effects of the transgenic. Likewise, if spraying of herbicides becomes more regular due to new cultivars, surrounding weeds could develop a resistance to the herbicide tolerant by the crop. This would cause an increase in herbicide dose or change in herbicide, as well as an increase in the amount and types of herbicides on crop plants. Ironically, chemical companies that sell weed killers are a driving force behind this research (Steinbrecher 1996 ).
Another issue is the uncertainty in whether the pest-resistant characteristic of these crops can escape to their weedy relatives causing resistant and increased weeds (Louda 1999 ). It is also possible that if insect-resistant plants cause increased death in one particular pest, it may decrease competition and invite minor pests to become a major problem. In addition, it could cause the pest population to shift to another plant population that was once unthreatened. These effects can branch out much further. A study of Bt crops showed that “beneficial insects, so named because they prey on crop pests, were also exposed to harmful quantities of Bt.” It was stated that it is possible for the effects to reach further up the food web to effect plants and animals consumed by humans (Brian 1999 ). Also, from a toxicological standpoint, further investigation is required to determine if residues from herbicide or pest resistant plants could harm key groups of organisms found in surrounding soil, such as bacteria, fungi, nematodes, and other microorganisms (Allison and Palma 1997 ).
The potential risks accompanied by disease resistant plants deal mostly with viral resistance. It is possible that viral resistance can lead to the formation of new viruses and therefore new diseases. It has been reported that naturally occurring viruses can recombine with viral fragments that are introduced to create transgenic plants, forming new viruses. Additionally, there can be many variations of this newly formed virus (Steinbrecher 1996 ).
Health risks associated with GM foods are concerned with toxins, allergens, or genetic hazards. The mechanisms of food hazards fall into three main categories (Conner and Jacobs 1999 ). They are inserted genes and their expression products, secondary and pleiotropic effects of gene expression and the insertional mutagenesis resulting from gene integration. With regards to the first category, it is not the transferred gene itself that would pose a health risk. It should be the expression of the gene and the affects of the gene product that are considered. New proteins can be synthesized that can produce unpredictable allergenic effects. For example, bean plants that were genetically modified to increase cysteine and methionine content were discarded after the discovery that the expressed protein of the transgene was highly allergenic (Butler and Reichhardt 1999 ). Due attention should be taken for foods engineered with genes from foods that commonly cause allergies, such as milk, eggs, nuts, wheat, legumes, fish, molluscs and crustacean (Maryanski 1997 ). However, since the products of the transgenic are usually previously identified, the amount and effects of the product can be assessed before public consumption. Also, any potential risk, immunological, allergenic, toxic or genetically hazardous, could be recognized and evaluated if health concerns arise. The available allergen data bases with details are shown in Table 1 .
Allergen databases (Kleter and Peijnenburg 2002 )
More concern comes with secondary and pleiotropic effects. For example, many transgenes encode an enzyme that alters biochemical pathways. This could cause an increase or decrease in certain biochemicals. Also, the presence of a new enzyme could cause depletion in the enzymatic substrate and subsequent build up of the enzymatic product. In addition, newly expressed enzymes may cause metabolites to diverge from one secondary metabolic pathway to another (Conner and Jacobs 1999 ). These changes in metabolism can lead to an increase in toxin concentrations. Assessing toxins is a more difficult task due to limitations of animal models. Animals have high variation between experimental groups and it is challenging to attain relevant doses of transgenic foods in animals that would provide results comparable to humans (Butler and Reichhardt 1999 ). Consequently, biochemical and regulatory pathways in plants are poorly understood.
Insertional mutagenesis can disrupt or change the expression of existing genes in a host plant. Random insertion can cause inactivation of endogenous genes, producing mutant plants. Moreover, fusion proteins can be made from plant DNA and inserted DNA. Many of these genes create nonsense products or are eliminated in crop selection due to incorrect appearance. However, of most concern is the activation or up regulation of silent or low expressed genes. This is due to the fact that it is possible to activate “genes that encode enzymes in biochemical pathways toward the production of toxic secondary compounds” (Conner and Jacobs 1999 ). This becomes a greater issue when the new protein or toxic compound is expressed in the edible portion of the plant, so that the food is no longer substantially equal to its traditional counterpart.
There is a great deal of unknowns when it comes to the risks of GM foods. One critic declared “foreign proteins that have never been in the human food chain will soon be consumed in large amounts”. It took us many years to realize that DDT might have oestrogenic activities and affect humans, “but we are now being asked to believe that everything is OK with GM foods because we haven’t seen any dead bodies yet” (Butler and Reichhardt 1999 ). As a result of the growing public concerns over GM foods, national governments have been working to regulate production and trade of GM foods.
Reports say that GM crops are grown over 160 million hectares in 29 countries, and imported by countries (including European ones) that don’t grow them. Nearly 300 million Americans, 1350 million Chinese, 280 million Brazilians and millions elsewhere regularly eat GM foods, directly and indirectly. Though Europeans voice major fears about GM foods, they permit GM maize cultivation. It imports GM soy meal and maize as animal feed. Millions of Europeans visit the US and South America and eat GM food.
Around three million Indians have become US citizens, and millions more go to the US for tourism and business and they will be eating GM foods in the USA. Indian activists claim that GM foods are inherently dangerous and must not be cultivated in India. Activists strongly opposed Bt cotton in India, and published reports claiming that the crop had failed in the field. At the same time farmers soon learned from experience that Bt cotton was very profitable, and 30 million rushed to adopt it. In consequence, India’s cotton production doubled and exports zoomed, even while using much less pesticide. Punjab farmers lease land at Rs 30,000 per acre to grow Bt cotton.
Public concerns-global scenario
In the late 1980s, there was a major controversy associated with GM foods even when the GMOs were not in the market. But the industrial applications of gene technology were developed to the production and marketing status. After words, the European Commission harmonized the national regulations across Europe. Concerns from the community side on GMOs in particular about its authorization have taken place since 1990s and the regulatory frame work on the marketing aspects underwent refining. Issues specifically on the use of GMOs for human consumption were introduced in 1997, in the Regulation on Novel Foods Ingredients (258/97/EC of 27 January 1997). This Regulations deals with rules for authorization and labelling of novel foods including food products made from GMOs, recognizing for the first time the consumer’s right to information and labelling as a tool for making an informed choice. The labelling of GM maize varieties and GM soy varieties that did not fall under this Regulation are covered by Regulation (EC 1139/98). Further legislative initiatives concern the traceability and labelling of GMOs and the authorization of GMOs in food and feed.
The initial outcome of the implementation of the first European directive seemed to be a settlement of the conflicts over technologies related to gene applications. By 1996, the second international level controversy over gene technology came up and triggered the arrival of GM soybeans at European harbours (Lassen et al. 2002 ). The GM soy beans by Monsanto to resist the herbicide represented the first large scale marketing of GM foods in Europe. Events such as commercialisation of GM maize and other GM modified commodities focused the public attention on the emerging biosciences, as did other gene technology applications such as animal and human cloning. The public debate on the issues associated with the GM foods resulted in the formation of many non-governmental organizations with explicit interest. At the same time there is a great demand for public participation in the issues about regulation and scientific strategy who expresses acceptance or rejection of GM products through purchase decisions or consumer boycotts (Frewer and Salter 2002 ).
Most research effort has been devoted to assessing people’s attitudes towards GM foods as a technology. Numerous “opinion poll”—type surveys have been conducted on national and cross-national levels (Hamstra 1998 ). Ethical concerns are also important, that a particular technology is in some way “tampering with nature”, or that unintended effects are unpredictable and thus unknown to science (Miles and Frewer 2001 ).
Consumer’s attitude towards GM foods
Consumer acceptance is conditioned by the risk that they perceive from introducing food into their consumption habits processed through technology that they hardly understand. In a study conducted in Spain, the main conclusion was that the introduction of GM food into agro-food markets should be accompanied by adequate policies to guarantee consumer safety. These actions would allow a decrease in consumer-perceived risk by taking special care of the information provided, concretely relating to health. For, the most influential factor in consumer-perceived risk from these foods is concern about health (Martinez-Poveda et al. 2009 ).
Tsourgiannis et al. ( 2011 ) conducted a study aimed to identify the factors that affect consumers purchasing behaviour towards food products that are free from GMO (GM Free) in a European region and more precisely in the Prefecture of Drama-Kavala-Xanthi. Field interviews conducted in a random selected sample consisted of 337 consumers in the cities of Drama, Kavala, Xanthi in 2009. Principal components analysis (PCA) was conducted in order to identify the factors that affect people in preferring consuming products that are GM Free. The factors that influence people in the study area to buy GM Free products are: (a) products’ certification as GM Free or organic products, (b) interest about the protection of the environment and nutrition value, (c) marketing issues and (d) price and quality. Furthermore, cluster and discriminant analysis identified two groups of consumers: (a) those influenced by the product price, quality and marketing aspects and (b) those interested in product’s certification and environmental protection (Tsourgiannis et al. 2011 ).
Snell et al. ( 2012 ) examined 12 long-term studies (of more than 90 days, up to 2 years in duration) and 12 multigenerational studies (from 2 to 5 generations) on the effects of diets containing GM maize, potato, soybean, rice, or triticale on animal health. They referenced the 90-day studies on GM feed for which long-term or multigenerational study data were available. Many parameters have been examined using biochemical analyses, histological examination of specific organs, hematology and the detection of transgenic DNA. Results from all the 24 studies do not suggest any health hazards and, in general, there were no statistically significant differences within parameters observed. They observed some small differences, though these fell within the normal variation range of the considered parameter and thus had no biological or toxicological significance. The studies reviewed present evidence to show that GM plants are nutritionally equivalent to their non-GM counterparts and can be safely used in food and feed.
GM foods: issues with respect to India
In a major setback to the proponents of GM technology in farm crops, the Parliamentary Committee on Agriculture in 2012 asked Indian government to stop all field trials and sought a bar on GM food crops such as Bt. brinjal. Raising the “ethical dimensions” of transgenics in agricultural crops, as well as studies of a long-term environmental and chronic toxicology impact, the panel noted that there were no significant socio-economic benefits to farmers.
Countries like India have great security concerns at the same time specific problems exist for small and marginal farmers. India could use a toxin free variety of the Lathyrus sativus grown on marginal lands and consumed by the very poor. GM mustard is a variety using the barnase-barstar-bar gene complex, an unstable gene construct with possible undesirable effects, to achieve male sterile lines that are used to make hybrid mustard varieties. In India we have good non-GM alternatives for making male sterile lines for hybrid production so the Proagro variety is of little use. Being a food crop, GM mustard will have to be examined very carefully. Even if there were to be benefits, they have to be weighed against the risks posed to human health and the environment. Apart from this, mustard is a cross-pollinating crop and pollen with their foreign genes is bound to reach non-GM mustard and wild relatives. We do not know what impact this will have. If GM technology is to be used in India, it should be directed at the real needs of Indian farmers, on crops like legumes, oilseeds and fodder and traits like drought tolerance and salinity tolerance.
Basmati rice and Darjeeling tea are perhaps India’s most easily identifiable premium products in the area of food. Basmati is highly prized rice, its markets are growing and it is a high end, expensive product in the international market. Like Champagne wine and truffles from France, international consumers treat it as a special, luxury food. Since rice is nutritionally a poor cereal, it is thought that addition of iron and vitamin A by genetic modification would increase the nutritional quality. So does it make any sense at all to breed a GM Basmati, along the lines of Bt Cotton? However, premium wine makers have outright rejected the notion of GM doctored wines that were designed to cut out the hangover and were supposed to be ‘healthier’. Premium products like special wines, truffles and Basmati rice need to be handled in a special, premium way (Sahai 2003 ).
Traceability of GMOs in the food production chain
Traceability systems document the history of a product and may serve the purpose of both marketing and health protection. In this framework, segregation and identity preservation systems allow for the separation of GM and non-GM products from “farm to fork”. Implementation of these systems comes with specific technical requirements for each particular step of the food processing chain. In addition, the feasibility of traceability systems depends on a number of factors, including unique identifiers for each GM product, detection methods, permissible levels of contamination, and financial costs. Progress has been achieved in the field of sampling, detection, and traceability of GM products, while some issues remain to be solved. For success, much will depend on the threshold level for adventitious contamination set by legislation (Miraglia et al. 2004 ).
Issues related to detection and traceability of GMOs is gaining interest worldwide due to the global diffusion and the related socio-economical implications. The interest of the scientific community into traceability aspects has also been increased simultaneously. Crucial factors in sampling and detection methodologies are the number of the GMOs involved and international agreement on traceability. The availability of reliable traceability strategies is very important and this may increase public trust in transparency in GMO related issues.
Heat processing methods like autoclaving and microwave heating can damage the DNA and reduce the level to detectable DNA. The PCR based methods have been standardised to detect such DNA in GM soybean and maize (Vijayakumar et al. 2009 ). Molecular methods such as multiplex and real time PCR methods have been developed to detect even 20 pg of genomic DNA in genetically modified EE-1 brinjal (Ballari et al. 2012 ).
DNA and protein based methods have been adopted for the detection and identification of GMOs which is relatively a new area of diagnostics. New diagnostic methodologies are also being developed, viz. the microarray-based methods that allow for the simultaneous identification of the increasing number of GMOs on the global market in a single sample. Some of these techniques have also been discussed for the detection of unintended effects of genetic modification by Cellini et al. ( 2004 ). The implementation of adequate traceability systems requires more than technical tools alone and is strictly linked to labelling constraints. The more stringent the labelling requirements, the more expensive and difficult the associated traceability strategies are to meet these requirements.
Both labelling and traceability of GMOs are current issues that are considered in trade and regulation. Currently, labelling of GM foods containing detectable transgenic material is required by EU legislation. A proposed package of legislation would extend this labelling to foods without any traces of transgenics. These new legislations would also impose labelling and a traceability system based on documentation throughout the food and feed manufacture system. The regulatory issues of risk analysis and labelling are currently harmonised by Codex Alimentarius. The implementation and maintenance of the regulations necessitates sampling protocols and analytical methodologies that allow for accurate determination of the content of GM organisms within a food and feed sample. Current methodologies for the analysis of GMOs are focused on either one of two targets, the transgenic DNA inserted- or the novel protein(s) expressed- in a GM product. For most DNA-based detection methods, the polymerase chain reaction is employed. Items that need consideration in the use of DNA-based detection methods include the specificity, sensitivity, matrix effects, internal reference DNA, availability of external reference materials, hemizygosity versus homozygosity, extra chromosomal DNA and international harmonisation.
For most protein-based methods, enzyme-linked immunosorbent assays with antibodies binding the novel protein are employed. Consideration should be given to the selection of the antigen bound by the antibody, accuracy, validation and matrix effects. Currently, validation of detection methods for analysis of GMOs is taking place. New methodologies are developed, in addition to the use of microarrays, mass spectrometry and surface plasmon resonance. Challenges for GMO detection include the detection of transgenic material in materials with varying chromosome numbers. The existing and proposed regulatory EU requirements for traceability of GM products fit within a broader tendency towards traceability of foods in general and, commercially, towards products that can be distinguished from one another.
Gene transfer studies in human volunteers
As of January 2009, there has only been one human feeding study conducted on the effects of GM foods. The study involved seven human volunteers who previously had their large intestines removed for medical reasons. These volunteers were provided with GM soy to eat to see if the DNA of the GM soy transferred to the bacteria that naturally lives in the human gut. Researchers identified that three of the seven volunteers had transgenes from GM soya transferred into the bacteria living in their gut before the start of the feeding experiment. As this low-frequency transfer did not increase after the consumption of GM soy, the researchers concluded that gene transfer did not occur during the experiment. In volunteers with complete digestive tracts, the transgene did not survive passage through intact gastrointestinal tract (Netherwood 2004 ). Other studies have found DNA from M13 virus, GFP and even ribulose-1, 5-bisphosphate carboxylase (Rubisco) genes in the blood and tissue of ingesting animals (Guertler et al. 2009 ; Brigulla and Wackernagel 2010 ).
Two studies on the possible effects of giving GM feed to animals found that there were no significant differences in the safety and nutritional value of feedstuffs containing material derived from GM plants (Gerhard et al. 2005 ; Beagle et al. 2006 ). Specifically, the studies noted that no residues of recombinant DNA or novel proteins have been found in any organ or tissue samples obtained from animals fed with GM plants (Nordlee 1996 ; Streit 2001 ).
Future developments
The GM foods have the potential to solve many of the world’s hunger and malnutrition problems, and to help protect and preserve the environment by increasing yield and reducing reliance upon synthetic pesticides and herbicides. Challenges ahead lie in many areas viz. safety testing, regulation, policies and food labelling. Many people feel that genetic engineering is the inevitable wave of the future and that we cannot afford to ignore a technology that has such enormous potential benefits.
Future also envisages that applications of GMOs are diverse and include drugs in food, bananas that produce human vaccines against infectious diseases such as Hepatitis B (Kumar et al. 2005 ), metabolically engineered fish that mature more quickly, fruit and nut trees that yield years earlier, foods no longer containing properties associated with common intolerances, and plants that produce new biodegradable plastics with unique properties (van Beilen and Yves 2008 ). While their practicality or efficacy in commercial production has yet to be fully tested, the next decade may see exponential increases in GM product development as researchers gain increasing access to genomic resources that are applicable to organisms beyond the scope of individual projects.
One has to agree that there are many opinions (Domingo 2000 ) about scarce data on the potential health risks of GM food crops, even though these should have been tested for and eliminated before their introduction. Although it is argued that small differences between GM and non-GM crops have little biological meaning, it is opined that most GM and parental line crops fall short of the definition of substantial equivalence. In any case, we need novel methods and concepts to probe into the compositional, nutritional, toxicological and metabolic differences between GM and conventional crops and into the safety of the genetic techniques used in developing GM crops if we want to put this technology on a proper scientific foundation and allay the fears of the general public. Considerable effort need to be directed towards understanding people’s attitudes towards this gene technology. At the same time it is imperative to note the lack of trust in institutions and institutional activities regarding GMOs and the public perceive that institutions have failed to take account of the actual concerns of the public as part of their risk management activities.
Contributor Information
A. S. Bawa, Email: [email protected]
K. R. Anilakumar, Email: [email protected]
- Allison S, Palma PM. Commercialization of transgenic plants: potential ecological risks. BioScience. 1997;47:86–96. doi: 10.2307/1313019. [ DOI ] [ Google Scholar ]
- Ballari VR, Martin A, Gowda LR (2012) Detection and identification of genetically modified EE-1 brinjal ( Solanum melongena ) by single, multiplex and SYBR® real-time PCR. J Sci Food Agric. doi:10.1002/jsfa.5764, Published online 22 June 2012 [ DOI ] [ PubMed ]
- Beagle JM, Apgar GA, Jones KL, Griswold KE, Radcliffe JS, Qiu X, Lightfoot DA, Iqbal MJ. The digestive fate of Escherichia coli glutamate dehydrogenase deoxyribonucleic acid from transgenic corn in diets fed to weanling pigs. J Anim Sci. 2006;84(3):597–607. doi: 10.2527/2006.843597x. [ DOI ] [ PubMed ] [ Google Scholar ]
- Berberich SA, Ream JE, Jackson TL, Wood R, Stipanovic R, Harvey P, Patzer S, Fuchs RL. The composition of insect-protected cottonseed is equivalent to that of conventional cottonseed. J Agric Food Chem. 1996;44:365–371. doi: 10.1021/jf950304i. [ DOI ] [ Google Scholar ]
- Bernstein IL, Bernstein JA, Miller M, Tierzieva S, Bernstein DI, Lummus Z, Selgrade MK, Doerfler DL, Seligy VL. Immune responses in farm workers after exposure to Bacillus thuringiensis pesticides. Environ Health Perspect. 1999;107:575–582. doi: 10.1289/ehp.99107575. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Brake J, Vlachos D. Evaluation of transgenic Event 176 “Bt” corn in broiler chicken. Poult Sci. 1998;77:648–653. doi: 10.1093/ps/77.5.648. [ DOI ] [ PubMed ] [ Google Scholar ]
- Brian H. Unintended effects of Bt crops. World Watch. 1999;12:9–10. [ Google Scholar ]
- Brigulla M, Wackernagel W. Molecular aspects of gene transfer and foreign DNA acquisition in prokaryotes with regard to safety issues. Appl Microbiol Biotechnol. 2010;86(4):1027–1041. doi: 10.1007/s00253-010-2489-3. [ DOI ] [ PubMed ] [ Google Scholar ]
- Burks AW, Fuchs RL. Assessment of the endogenous allergens in glyphosate-tolerant and commercial soybean varieties. J Allergy Clin Immunol. 1995;96:1008–1010. doi: 10.1016/S0091-6749(95)70243-1. [ DOI ] [ PubMed ] [ Google Scholar ]
- Butler T, Reichhardt T. Long-term effect of GM crops serves up food for thought. Nature. 1999;398(6729):651–653. doi: 10.1038/19348. [ DOI ] [ PubMed ] [ Google Scholar ]
- Cellini F, Chesson A, Colquhoun I, Constable A, Davies HV, Engel KH, Gatehouse AMR, Karenlampi S, Kok EJ, Leguay JJ, Lehasranta S, Noteborn HPJM, Pedersen J, Smith M. Unintended effects and their detection in genetically modified crops. Food Chem Toxicol. 2004;42:1089–1125. doi: 10.1016/j.fct.2004.02.003. [ DOI ] [ PubMed ] [ Google Scholar ]
- Chapman MD. Allergen nomenclature. In: Lockey RF, Dennis Ledford K, editors. Allergens and allergen immunotherapy. 4. New York: Informa Healthcare; 2008. pp. 47–58. [ Google Scholar ]
- Clive J (1996) Global review of the field testing and commercialization of transgenic plants: 1986 to 1995. The International Service for the Acquisition of Agri-biotech Applications. http://www.isaaa.org/kc/Publications/pdfs/isaaabriefs/Briefs%201.pdf . Retrieved on 17 July 2010
- Clive J. Global status of commercialized Biotech/GM crops. ISAAA Briefs 43. Ithaca: International Service for the Acquisition of Agri-biotech Applications; 2011. [ Google Scholar ]
- Conner AJ, Jacobs JME. Genetic engineering of crops as potential source of genetic hazard in the human diet. Mutat Res Genet Toxicol Environ Mutagen. 1999;443:223–234. doi: 10.1016/S1383-5742(99)00020-4. [ DOI ] [ PubMed ] [ Google Scholar ]
- Crevel RWR, Lerkhof MAT, Koning MMG. Allergenicity of refined vegetable oils. Food Chem Toxicol. 2000;38(4):385–393. doi: 10.1016/S0278-6915(99)00158-1. [ DOI ] [ PubMed ] [ Google Scholar ]
- Deisingh AK, Badrie N. Detection approaches for genetically modified organisms in foods. Food Res Int. 2005;38:639–649. doi: 10.1016/j.foodres.2005.01.003. [ DOI ] [ Google Scholar ]
- Domingo JL. Health risks of genetically modified foods: many opinions but few data. Science. 2000;288:1748–1749. doi: 10.1126/science.288.5472.1748. [ DOI ] [ PubMed ] [ Google Scholar ]
- Ewen SWB, Pusztai A. Effects of diets containing genetically modified potatoes expressing Galanthus nivalis lectin on rat small intestine. Lancet. 1999;354:1353–1354. doi: 10.1016/S0140-6736(98)05860-7. [ DOI ] [ PubMed ] [ Google Scholar ]
- Fares NH, El-Sayed AK. Fine structural changes in the ileum of mice fed on delta-endotoxin-treated potatoes and transgenic potatoes. Nat Toxins. 1998;6:219–233. doi: 10.1002/(SICI)1522-7189(199811/12)6:6<219::AID-NT30>3.0.CO;2-K. [ DOI ] [ PubMed ] [ Google Scholar ]
- Frewer LI, Salter B. Public attitudes, scientific advice and the politics of regulatory policy the case of BSE. Sci Public Policy. 2002;29:137–145. doi: 10.3152/147154302781781092. [ DOI ] [ Google Scholar ]
- Gerhard F, Andrew C, Karen A. Animal nutrition with feeds from genetically modified plants. Arch Anim Nutr. 2005;59:1–40. doi: 10.1080/17450390512331342368. [ DOI ] [ PubMed ] [ Google Scholar ]
- Guertler P, Paul V, Albrech C, Meyer HH. Sensitive and highly specific quantitative real-time PCR and ELISA for recording a potential transfer of novel DNA and Cry1Ab protein from feed into bovine milk. Anal Bioanal Chem. 2009;393:1629–1638. doi: 10.1007/s00216-009-2667-2. [ DOI ] [ PubMed ] [ Google Scholar ]
- Hamer H, Scuse T (2010) National Agricultural Statistics Service (NASS), Agricultural Statistics Board, US Department of Agriculture. Acreage report, NY
- Hammond BG, Vicini JL, Hartnell GF, Naylor MW, Knight CD, Robinson EH, Fuchs RL, Padgette SR. The feeding value of soybeans fed to rats, chickens, catfish and dairy cattle is not altered by genetic incorporation of glyphosate tolerance. J Nutr. 1996;126:717–727. doi: 10.1093/jn/126.3.717. [ DOI ] [ PubMed ] [ Google Scholar ]
- Hamstra A (1998) Public opinion about Biotechnology. A survey of surveys. European Federation of Biotechnology, The Hague
- Harrison LA, Bailey MR, Naylor MW, Ream JE, Hammond BG, Nida DL, Burnette BL, Nickson TE, Mitsky TA, Taylor ML, Fuchs RL, Padgette SR. The expressed protein in glyphosate-tolerant soybean, 5-enolpyruvylshikimate-3-phosphate synthase from Agrobacterium sp. strain CP4, is rapidly digested in vitro and is not toxic to acutely gavaged mice. J Nutr. 1996;126:728–740. doi: 10.1093/jn/126.3.728. [ DOI ] [ PubMed ] [ Google Scholar ]
- Hashimoto W, Momma K, Katsube T, Ohkawa Y, Ishige T, Kito M, Utsumi S, Murata K. Safety assessment of genetically engineered potatoes with designed soybean glycinin: compositional analyses of the potato tubers and digestibility of the newly expressed protein in transgenic potatoes. J Sci Food Agric. 1999;79:1607–1612. doi: 10.1002/(SICI)1097-0010(199909)79:12<1607::AID-JSFA408>3.0.CO;2-T. [ DOI ] [ Google Scholar ]
- Hashimoto W, Momma K, Yoon HJ, Ozawa S, Ohkawa Y, Ishige T, Kito M, Utsumi S, Murata K. Safety assessment of transgenic potatoes with soybean glycinin by feeding studies in rats. Biosci Biotechnol Biochem. 1999;63:1942–1946. doi: 10.1271/bbb.63.1942. [ DOI ] [ PubMed ] [ Google Scholar ]
- IRDC (1998) Alliance for biointegrity. http://www.biointegrity.org including Calgene FLAVR SAVR™ tomato report, pp 1–604; International Research and Development Corp. first test report, pp 1736–1738; Conclusions of the expert panel regarding the safety of the FLAVR SAVR™ tomato, ENVIRON, Arlington VA, USA pp 2355–2382; Four week oral (intubation) toxicity study in rats by IRDC, pp 2895–3000
- Ivanciuc O, Schein CH, Braun W. SDAP: database and computational tools for allergenic proteins. Nucleic Acids Res. 2003;31:359–362. doi: 10.1093/nar/gkg010. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Joana C, Isabel M, Joana SA, Oliveira MBPP. Monitoring genetically modified soybean along the industrial soybean oil extraction and refining processes by polymerase chain reaction techniques. Food Res Int. 2010;43:301–306. doi: 10.1016/j.foodres.2009.10.003. [ DOI ] [ Google Scholar ]
- Johnson SR. Quantification of the impacts on US Agriculture of Biotechnology-Derived Crops Planted in 2006. Washington DC: National Centre for Food and Agricultural Policy; 2008. [ Google Scholar ]
- Kleter GA, Peijnenburg AACM. Screening of transgenic proteins expressed in transgenic food crops for the presence of short amino acid sequences identical to potential, IgE-binding linear epitopes of allergens. BMC Struct Biol. 2002;2:8–19. doi: 10.1186/1472-6807-2-8. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Kumar GBS, Ganapathi TR, Revathi CJ, Srinivas L, Bapat VA. Expression of hepatitis B surface antigen in transgenic banana plants. Planta. 2005;222:484–493. doi: 10.1007/s00425-005-1556-y. [ DOI ] [ PubMed ] [ Google Scholar ]
- Lack G. Clinical risk assessment of GM foods. Toxicol Lett. 2002;127:337–340. doi: 10.1016/S0378-4274(01)00517-3. [ DOI ] [ PubMed ] [ Google Scholar ]
- La Mura M, Allnutt TR, Greenland A, Mackay LD. Application of QUIZ for GM quantification in food. Food Chem. 2011;125:1340–1344. doi: 10.1016/j.foodchem.2010.10.002. [ DOI ] [ Google Scholar ]
- Lappe MA, Bailey EB, Childress C, Setchell KDR. Alterations in clinically important phytoestrogens in genetically modified, herbicide-tolerant soybeans. J Med Food. 1999;1:241–245. doi: 10.1089/jmf.1998.1.241. [ DOI ] [ Google Scholar ]
- Lassen J, Allansdottir A, Liakoupulos M, Olsson A, Mortensen AT. Testing times: the reception of round-up ready soya in Europe. In: Bauer M, Gaskell G, editors. Biotechnology—the making of a global controversy. Cambridge: Cambridge University Press; 2002. pp. 279–312. [ Google Scholar ]
- Louda SM (1999) Insect Limitation of weedy plants and its ecological implications. In: Traynor PL, Westwood J H (eds) Proceedings of a workshop on: ecological effects of pest resistance genes in managed ecosystems. Information Systems for Biotechnology. Blacksburg, Virginia, pp 43–48, http://www.isb.vt.edu
- Mari A, Riccioli D. The allergome web site—a database of allergenic molecules. Aim, structure, and data of a web-based resource. J Allergy Clin Immunol. 2004;113:S301. [ Google Scholar ]
- Martinez-Poveda A, Molla-Bauza MB, Gomis FJC, Martinez LMC. Consumer-perceived risk model for the introduction of genetically modified food in Spain. Food Policy. 2009;34:519–528. doi: 10.1016/j.foodpol.2009.08.001. [ DOI ] [ Google Scholar ]
- Maryanski JH. Bioengineered foods: will they cause allergic reactions? NY: U.S. Food and Drug Administration (FDA)/Centre for Food Safety and Applied Nutrition (CFSAN); 1997. [ Google Scholar ]
- Metcalf DD, Astwood JD, Townsend R, Sampson HA, Taylor SL, Fuchs RL (1996) Assessment of the allergenic potential of foods derived from genetically engineered crop plants. In: Crit Rev Food Sci Nutr 36(S):S165–S186. CRC, Boca Raton [ DOI ] [ PubMed ]
- Miles S, Frewer LI. Investigating specific concerns about different food hazards—higher and lower order attributes. Food Qual Prefer. 2001;12:47–61. doi: 10.1016/S0950-3293(00)00029-X. [ DOI ] [ Google Scholar ]
- Miraglia M, Berdal K, Brera C, Corbisier P, Holst-jensen A, Kok E, Marvin H, Schimmel H, Rentsch J, van Rie J, Zagon J. Detection and traceability of genetically modified organisms in the food production chain. Food Chem Toxicol. 2004;42:1157–1180. doi: 10.1016/j.fct.2004.02.018. [ DOI ] [ PubMed ] [ Google Scholar ]
- Momma K, Hashimoto W, Ozawa S, Kawai S, Katsube T, Takaiwa F, Kito M, Utsumi S, Murata K. Quality and safety evaluation of genetically engineered rice with soybean glycinin: analyses of the grain composition and digestibility of glycinin in transgenic rice. Biosci Biotechnol Biochem. 1999;63:314–318. doi: 10.1271/bbb.63.314. [ DOI ] [ PubMed ] [ Google Scholar ]
- Nakamura R, Matsuda T. Rice allergenic protein and molecular-genetic approach for hypoallergenic rice. Biosci Biotechnol Biochem. 1996;60:1215–1221. doi: 10.1271/bbb.60.1215. [ DOI ] [ PubMed ] [ Google Scholar ]
- Netherwood T. Assessing the survival of transgenic plant DNA in the human gastrointestinal tract. Nat Biotechnol. 2004;22:204–209. doi: 10.1038/nbt934. [ DOI ] [ PubMed ] [ Google Scholar ]
- Nordlee JA. Identification of Brazil-Nut allergen in transgenic soybeans. New Engl J Med. 1996;334:688–692. doi: 10.1056/NEJM199603143341103. [ DOI ] [ PubMed ] [ Google Scholar ]
- Nordlee JA, Taylor SL, Townsend JA, Thomas LA. Identification of a Brazil nut allergen in transgenic soybean. New Engl J Med. 1996;334:688–692. doi: 10.1056/NEJM199603143341103. [ DOI ] [ PubMed ] [ Google Scholar ]
- Noteborn HPJM, Bienenmann-Ploum ME, van den Berg JHJ, Alink GM, Zolla L, Raynaerts A, Pensa M, Kuiper HA. Safety assessment of the Bacillus thuringiensis insecticidal crystal protein CRYIA(b) expressed in transgenic tomatoes. In: Engel KH, Takeoka GR, Teranishi R, editors. ACS Symp series 605 Genetically modified foods—safety issues. Washington, D.C: American Chemical Society; 1995. pp. 135–147. [ Google Scholar ]
- Novak WK, Haslberger AG. Substantial equivalence of antinutrients and inherent plant toxins in genetically modified novel foods. Food Chem Toxicol. 2000;38:473–483. doi: 10.1016/S0278-6915(00)00040-5. [ DOI ] [ PubMed ] [ Google Scholar ]
- O’Neil C, Reese G, Lehrer SB. Allergenic potential of recombinant food proteins. Allergy Clin Immunol Int. 1998;10:5–9. [ Google Scholar ]
- Padgette SR, Taylor NB, Nida DL, Bailey MR, MacDonald J, Holden LR, Fuchs RL. The composition of glyphosate-tolerant soybean seeds is equivalent to that of conventional soybeans. J Nutr. 1996;126:702–716. doi: 10.1093/jn/126.3.702. [ DOI ] [ PubMed ] [ Google Scholar ]
- Pusztai A (2001) Safety tests on commercial crops. American Institute of Biological Sciences. actionbioscience.org, http://www.actionbioscience.org/biotech/pusztai.html viewed 2 March 2010
- Pusztai A, Ewen SWB, Grant G, Peumans WJ, van Damme EJM, Rubio L, Bardocz S. Relationship between survival and binding of plant lectins during small intestinal passage and their effectiveness as growth factors. Digestion. 1990;46(suppl 2):308–316. doi: 10.1159/000200402. [ DOI ] [ PubMed ] [ Google Scholar ]
- Pusztai A, Grant G, Bardocz S, Alonso R, Chrispeels MJ, Schroeder HE, Tabe LM, Higgins TJV. Expression of the insecticidal bean alpha-amylase inhibitor transgene has minimal detrimental effect on the nutritional value of peas fed to rats at 30 % of the diet. J Nutr. 1999;129:1597–1603. doi: 10.1093/jn/129.8.1597. [ DOI ] [ PubMed ] [ Google Scholar ]
- Redenbaugh K, Hiatt W, Martineau B, Kramer M, Sheehy R, Sanders R, Houck C, Emlay D (1992) Safety assessment of genetically engineered fruits and vegetables: a case study of the Flavr Savr Tomato. CRC Press, Boca Raton
- Sahai S. Genetically modified crops: issues for India. Fin Agric. 2003;35:7–11. [ Google Scholar ]
- Snell C, Bernheim A, Bergé J-B, Kuntz M, Pascal G, Paris A, Agnès ER. Assessment of the health impact of GM plant diets in long-term and multigenerational animal feeding trials: a literature review. Food Chem Toxicol. 2012;50:1134–1148. doi: 10.1016/j.fct.2011.11.048. [ DOI ] [ PubMed ] [ Google Scholar ]
- Steinbrecher RA. From green to gene evolution: the environmental risks of genetically engineered crops. Ecologist. 1996;26:273–281. [ Google Scholar ]
- Streit L. Association of the Brazil nut protein gene and Kunitz trypsin inhibitor alleles with soybean protease inhibitor activity and agronomic traits. Crop Sci. 2001;41:1757–1760. doi: 10.2135/cropsci2001.1757. [ DOI ] [ Google Scholar ]
- Taylor NB, Fuchs RL, MacDonald J, Shariff AB, Padgette SR. Compositional analysis of glyphosate-tolerant soybeans treated with glyphosate. J Agric Food Chem. 1999;47:4469–4473. doi: 10.1021/jf990056g. [ DOI ] [ PubMed ] [ Google Scholar ]
- Teshima R, Akiyama H, Okunuki H, Sakushima J-i, Goda Y, Onodera H, Sawada J-i, Toyoda M. Effect of GM and non-GM soybeans on the immune system of BN rats and B10A mice. J Food Hyg Soc Jpn. 2000;41:188–193. doi: 10.3358/shokueishi.41.188. [ DOI ] [ Google Scholar ]
- Tsourgiannis L, Karasavvoglou A, Florou G. Consumers’ attitudes towards GM free products in a European region. The case of the Prefecture of Drama-Kavala-Xanthi in Greece. Appetite. 2011;57:448–458. doi: 10.1016/j.appet.2011.06.010. [ DOI ] [ PubMed ] [ Google Scholar ]
- van Beilen JB, Yves P. Harnessing plant biomass for biofuels and biomaterials: production of renewable polymers from crop plants. Plant J. 2008;54(4):684–701. doi: 10.1111/j.1365-313X.2008.03431.x. [ DOI ] [ PubMed ] [ Google Scholar ]
- Vazquez-Padron RI, Moreno-Fierros L, Neri-Bazan L, Martinez-Gil AF, de la Riva GA, Lopez-Revilla R. Characterization of the mucosal and sytemic immune response induced by Cry1Ac protein from Bacillus thuringiensis HD 73 in mice. Braz J Med Biol Res. 2000;33:147–155. doi: 10.1590/S0100-879X2000000200002. [ DOI ] [ PubMed ] [ Google Scholar ]
- Vijayakumar KR, Martin A, Gowda LR, Prakash V. Detection of genetically modified soya and maize: impact of heat processing. Food Chem. 2009;117:514–521. doi: 10.1016/j.foodchem.2009.04.028. [ DOI ] [ Google Scholar ]
- Xiumin W, Da T, Qingfeng G, Fang T, Jianhua W. Detection of Roundup Ready soybean by loop-mediated isothermal amplification combined with a lateral-flow dipstick. Food Control. 2012;29:213–220. [ Google Scholar ]
- View on publisher site
- PDF (294.7 KB)
- Collections
Similar articles
Cited by other articles, links to ncbi databases.
- Download .nbib .nbib
- Format: AMA APA MLA NLM
Add to Collections
- Open access
- Published: 13 January 2022
Evaluation of adverse effects/events of genetically modified food consumption: a systematic review of animal and human studies
- Chen Shen 1 ,
- Xiang-Chang Yin 2 ,
- Bo-Yang Jiao 3 ,
- Jing Li 4 ,
- Peng Jia 5 ,
- Xiao-Wen Zhang 1 ,
- Xue-Hao Cheng 6 ,
- Jian-Xin Ren 6 ,
- Hui-Di Lan 7 ,
- Wen-Bin Hou 1 ,
- Min Fang 1 ,
- Yu-Tong Fei 1 ,
- Nicola Robinson 1 , 8 &
- Jian-Ping Liu ORCID: orcid.org/0000-0002-0320-061X 1 , 9
Environmental Sciences Europe volume 34 , Article number: 8 ( 2022 ) Cite this article
55k Accesses
17 Citations
124 Altmetric
Metrics details
A systematic review of animal and human studies was conducted on genetically modified (GM) food consumption to assess its safety in terms of adverse effects/events to inform public concerns and future research.
Seven electronic databases were searched from January 1st 1983 till July 11th 2020 for in vivo, animal and human studies on the incidence of adverse effects/events of GM products consumption. Two authors independently identified eligible studies, assessed the study quality, and extracted data on the name of the periodical, author and affiliation, literature type, the theme of the study, publication year, funding, sample size, target population characteristics, type of the intervention/exposure, outcomes and outcome measures, and details of adverse effects/events. We used the Chi-square test to compare the adverse event reporting rates in articles funded by industry funding, government funding or unfunded articles.
One crossover trial in humans and 203 animal studies from 179 articles met the inclusion criteria. The study quality was all assessed as being unclear or having a high risk of bias. Minor illnesses were reported in the human trial. Among the 204 studies, 59.46% of adverse events (22 of 37) were serious adverse events from 16 animal studies (7.84%). No significant differences were found in the adverse event reporting rates either between industry and government funding ( χ 2 = 2.286, P = 0.131), industry and non-industry funding ( χ 2 = 1.761, P = 0.185) or funded and non-funded articles ( χ 2 = 0.491, P = 0.483). We finally identified 21 GM food-related adverse events involving 7 GM events (NK603 × MON810 maize, GTS 40-3-2 soybean, NK603 maize, MON863 maize, MON810 maize, MON863 × MON810 × NK603 maize and GM Shanyou 63 rice), which had all been on regulatory approval in some countries/regions.
Serious adverse events of GM consumption include mortality, tumour or cancer, significant low fertility, decreased learning and reaction abilities, and some organ abnormalities. Further clinical trials and long-term cohort studies in human populations, especially on GM food-related adverse events and the corresponding GM events, are still warranted. It suggests the necessity of labelling GM food so that consumers can make their own choice.
Introduction
Genetic modification is defined as introducing transgene(s) with desired traits into the recipient organism’s genome by recombinant deoxyribonucleic acid (DNA) technology, and therefore it does not occur naturally [ 1 , 2 , 3 ]. Genetically modified (GM) crops are thought to address food security, sustainability and climate change solutions by improving crop yields, conserving biodiversity, providing a better environment in terms of the insect-resistant and herbicide-tolerant traits, reducing CO 2 emissions and helping alleviate poverty through uplifting the economic situation [ 4 ]. Insect-resistant and herbicide-tolerant traits were first introduced into four types of crop, canola, cotton, maize and soybeans, at the beginning of GM production [ 5 ]. At present, the mainstream characteristics of new crops still pursue higher-yielding, more nutritious, pest- and disease-resistant and climate-smart to meet future demand for a yield increase of major crops such as wheat, rice and corn, due to the growing population [ 6 ].
Since 1996, the first year of commercialization of GM crops, 70 countries had adopted GM crops until 2018, including 26 countries that cumulatively planted 2.5 billion hectares of GM crops and an additional 44 countries that imported GM crops. During the 27 years (1992 to 2018), 4349 approvals for 387 GM events from 27 GM crops were granted by 70 countries involving 2063 for food (when the direct consumers are mainly humans), 1461 for feed (the products only intended for animal consumption) use and 825 for environmental release or cultivation [ 4 , 7 ]. The major agricultural product exporting countries like the U.S.A., Brazil and Argentina show over 90% adoption of biotech crops [ 4 ]. For GM animal products, biotech salmon, considered to be the first genetically engineered animal for human consumption, was approved by the United States Department of Agriculture and Food & Drug Administration in 2015 [ 8 ]. In addition, it is illegal to grow major GM food crops in China while there are substantial investments in biotechnology research and GM maize, soybeans, and canola are allowed to import and eat [ 9 ].
Genetically modified food, however, is an example of the controversial relation between the inherent uncertainty of the scientific approach and the need of consumers to use products resulting from scientific developments thought to be safe [ 10 ]. Significant health risks have not been reported in peer-reviewed studies on GM food safety/security, which may cause some publication bias [ 11 ] but with a few exceptions, like the most famous “Monarch Butterfly controversy” [ 12 ], "Pusztai case" [ 13 ] and the "Séralini case" [ 14 ]. Unexpected effects of GM crops were reported in these studies, occupying an important place in the pages of scientific journals. Nevertheless, the above controversies severely impacted the public image, leading to full or partial bans in 38 countries including the European Union [ 15 ].
The complexity of risk evaluation is shown in these conflicting results, and concerns about the citizen-consumers have been raised against GM food [ 10 ]. Of most concern, aroused from the controversial events and some research results, is the potential of carcinogenesis, teratogenesis [ 16 ], lethal effects and adverse influences on fertility. GM agriculture is now widely discussed in both positive and negative frames and currently serves as a hotbed of debate in the public and policymakers. Although there are some reports and evidence from human and animal studies on the potential health effects of GM food/feed, the evidence is not conclusive and public concerns have not been resolved.
We aimed to conduct a systematic review of animal and human studies on GM food consumption to assess its safety in terms of adverse effects/events to inform public concerns and future research.
This study was a systematic review of previously published studies, conducted and reported in adherence with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) [ 17 ] guideline.
Search strategy
China National Knowledge Infrastructure (CNKI), Wanfang, VIP Database, Chinese Biomedical Database (SinoMed), PubMed, the Cochrane Library and Embase databases were searched from January, 1st, 1983 till July, 11th, 2020, using a predefined search strategy (Additional file 1 : Appendix S1). Reference lists of retrieved articles were also searched.
Eligibility criteria
Based on the evidence pyramid proposed by the Medical Center of State University of New York in 2001, we determined the type of research we included in the study. For a comprehensive evaluation of the literature, all in vivo animal studies and human studies (cross-sectional studies, case reports, case series, case–control studies, case–crossover studies, cohort studies, controlled clinical trials, including randomized trials, quasi-randomized trials and non-randomized trials) in multiple languages were included. Animal studies in all fields were included, that is, they could be clinical, agricultural and animal husbandry, veterinary medicine, life sciences, etc. Field studies were excluded.
The study population in animal studies was applied with inclusion criteria based on the categorization approach that highlights the actual use of them: laboratory animals and economical animals (livestock and aquatilia) were included, with no prespecified limitations on age, population, species/races, health status or others. Interventions/exposures of the genetically modified animal/plant/microorganism products included for animal/human ingestion referred to GM food, GM food ingredients and GM feed, regardless of their dosage or duration. The GM strain (line) and GM event were not limited. There was no restriction on whether controls were or were not included. The studies were excluded if they focused on the effects of GM food/feed on secondary or multilevel consumers in the food chain where GM food/feed was only consumed by primary consumers in the predator relationships. For instance, if non-GM fishes were fed with diet containing GM ingredients and then the fish was fed to the experimental cats, the study was excluded.
Outcomes focused on the incidence of adverse effects or adverse events in GM food/feed consumption, including primary outcomes on carcinogenesis, teratogenesis, lethal effect (all-cause mortality) and reproduction and secondary outcomes on other biomarkers were included. Toxicity studies of general toxicity studies (acute, sub-acute, sub-chronic, chronic and carcinogenicity toxicity studies) and specific toxicity studies (genotoxicity, reproductive and developmental toxicity, immunotoxicity and other toxicology studies) were included. Mortality in pups before weaning was considered as an outcome of reproductive toxicity but not as a lethal effect. Outcomes of adverse events in laboratory testing would not be included only when they could indicate tissue or organ toxicity. Outcomes of adverse events in breeding performance in animal husbandry studies, which focused on the economic benefits of the animal products, were included and these indicators were regarded as reproduction biomarkers in this research.
Outcomes of adverse events on growth performance, carcass traits, meat and fur production performance and meat quality for economic benefit evaluation of live stocks were excluded, of which the indicators included final body weight, weight gain, feed to gain ratio, half-eviscerated weight, eviscerated weight, percentage of eviscerated yield and muscle lean meat, sebum rate in some parts of the body, etc. Studies on the insecticidal effect of insect-resistant GM feed and outcomes of adverse events in gene fragments residual in the digestive tract were excluded. Besides, duplicate publications, studies with duplicate statistics, or references devoid of necessary information of participants, sample size, interventions/exposures or results were excluded.
Study selection and data extraction
Titles and abstracts of the retrieved articles were reviewed by 6 researchers in pair (C Shen, XC Yin, BY Jiao, J Peng, YZ Li, XH Cheng). 6 authors (C Shen, XC Yin, BY Jiao, JX Ren, J Li and XW Zhang) independently reviewed the full texts to identify the studies meeting eligibility criteria and then 8 researchers in pair (C Shen, XC Yin, BY Jiao, J Li, P Jia, XW Zhang, XH Cheng and JX Ren) independently extracted data from the included studies according to a predesignated extraction table. The discrepancies were resolved through consensus and if necessary, arbitrated by another author (JP Liu).
We extracted the name of the periodical, author and affiliation, literature type, the theme of the study, publication year, funding, sample size, target population characteristics, type of the intervention/exposure, outcomes and outcome measures. For those studies in which adverse effects/events occurred, details of interventions/exposures and control conditions (if any), dosage, duration, number of the generation, and the results were extracted.
Quality assessment
The methodological quality for animal studies was assessed, using criteria from the SYRCLE’s risk of bias tool for animal studies. The quality of animal studies was categorized into low risk of bias, unclear risk of bias, or high risk of bias according to the risk for each important outcome within included studies, including the adequacy of generation of the sequence generation, baseline characteristics, allocation concealment, random housing, blinding (performance bias), random outcome assessment, blinding (detection bias), incomplete outcome data, selective outcome reporting, or other sources of bias. The judgment of other risk of bias was based on whether there were contamination (pooling drugs), inappropriate influence of funders, unit of analysis errors, design-specific risks of bias or new animals added to the control and experimental groups to replace drop-outs from the original population.
Statistical synthesis and analyses
Statistical analyses were carried out using Microsoft Excel 2016 and SPSS 20.0. The findings were reported mainly in two parts, characteristics of the included studies and detailed information on the studies in which adverse effects/events occurred. Initially, descriptive statistics, frequencies, and percentages were calculated to summarize the data. Subsequently, studies that evaluated similar populations, interventions, controls (if any) and outcomes were pooled using a random-effects meta-analysis, and data from other studies were presented in tables and described in a narrative summary. The incidence of adverse events reported in articles funded by industry funding, government funding or unfunded articles were, respectively, counted and the Chi-square test was used for the comparisons.
Besides, we figured the incidence of serious adverse events (SAEs) by percentage. With reference to the Food and Drug Administration’s definition [ 18 ], our study defined SAEs as death, life-threatening, hospitalization (initial or prolonged), disability or permanent change, disruption, impairment or damage in a body function or structure (including cancer or tumour), in physical activities or quality of life, congenital anomaly or birth defect in the newborn child or pups, infertility or significant low in the number of deliveries or live birth rate than the non-GM commercial, conventional or blank controls, and an event resulting in intervention/treatment to prevent permanent impairment, damage or to prevent one of the other outcomes.
Meanwhile, the adverse events which cannot be ruled out that it has nothing to do with GM food (hereinafter abbreviated as GM food-related adverse events) were identified and the percentages under each outcome were calculated.
Description of studies
The flow diagram of the literature selection is shown in Fig. 1 . A total of 9668 records were identified, including 9584 from the initial search through seven databases and 84 from other sources. After removal of duplicates and exclusion of references by reading titles and abstracts, 455 full-text articles were screened and 276 references were excluded with reasons (seen in the flow chart). Finally, 204 studies from 179 articles [ 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 , 57 , 58 , 59 , 60 , 61 , 62 , 63 , 64 , 65 , 66 , 67 , 68 , 69 , 70 , 71 , 72 , 73 , 74 , 75 , 76 , 77 , 78 , 79 , 80 , 81 , 82 , 83 , 84 , 85 , 86 , 87 , 88 , 89 , 90 , 91 , 92 , 93 , 94 , 95 , 96 , 97 , 98 , 99 , 100 , 101 , 102 , 103 , 104 , 105 , 106 , 107 , 108 , 109 , 110 , 111 , 112 , 113 , 114 , 115 , 116 , 117 , 118 , 119 , 120 , 121 , 122 , 123 , 124 , 125 , 126 , 127 , 128 , 129 , 130 , 131 , 132 , 133 , 134 , 135 , 136 , 137 , 138 , 139 , 140 , 141 , 142 , 143 , 144 , 145 , 146 , 147 , 148 , 149 , 150 , 151 , 152 , 153 , 154 , 155 , 156 , 157 , 158 , 159 , 160 , 161 , 162 , 163 , 164 , 165 , 166 , 167 , 168 , 169 , 170 , 171 , 172 , 173 , 174 , 175 , 176 , 177 , 178 , 179 , 180 , 181 , 182 , 183 , 184 , 185 , 186 , 187 , 188 , 189 , 190 , 191 , 192 , 193 , 194 , 195 , 196 , 197 ] (153 journal articles, 22 dissertations, 3 conference proceedings and 1 unpublished report) were included in data synthesis, since there were more than one study conducted in each of the 2 included dissertations [ 107 , 127 ], 11 journal articles [ 19 , 33 , 35 , 63 , 67 , 88 , 102 , 118 , 132 , 172 , 184 ] and 1 unpublished report [ 32 ]. The included studies were of 203 in vivo animal studies and 1 crossover trial [ 97 ] in humans.
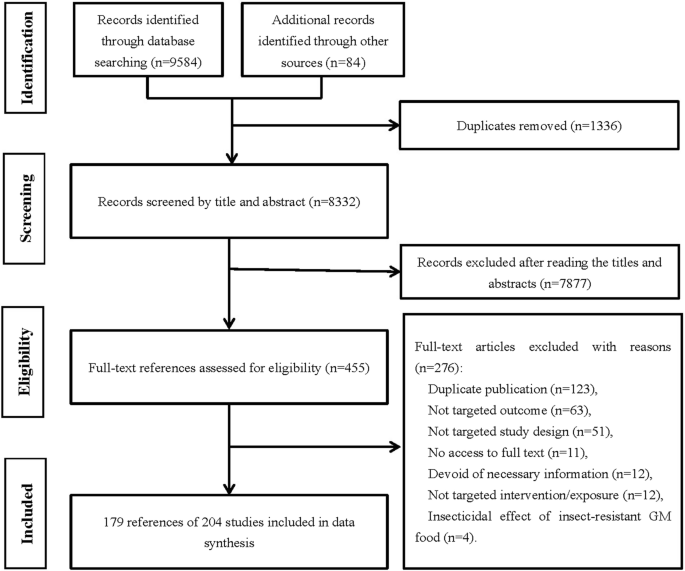
The flow of literature search and selection of studies on the safety of GM food
Study characteristics
Of the 179 included articles, 94 were in English [ 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 , 57 , 58 , 59 , 60 , 61 , 62 , 63 , 64 , 65 , 66 , 67 , 68 , 69 , 70 , 71 , 72 , 73 , 74 , 75 , 76 , 77 , 78 , 79 , 80 , 81 , 82 , 83 , 84 , 85 , 86 , 87 , 88 , 89 , 90 , 91 , 92 , 93 , 94 , 95 , 96 , 97 , 98 , 99 , 100 , 101 , 102 , 103 , 104 , 105 , 106 , 107 , 108 , 109 , 110 , 111 , 112 ], 83 were published in Chinese [ 113 , 114 , 115 , 116 , 117 , 118 , 119 , 120 , 121 , 122 , 123 , 124 , 125 , 126 , 127 , 128 , 129 , 130 , 131 , 132 , 133 , 134 , 135 , 136 , 137 , 138 , 139 , 140 , 141 , 142 , 143 , 144 , 145 , 146 , 147 , 148 , 149 , 150 , 151 , 152 , 153 , 154 , 155 , 156 , 157 , 158 , 159 , 160 , 161 , 162 , 163 , 164 , 165 , 166 , 167 , 168 , 169 , 170 , 171 , 172 , 173 , 174 , 175 , 176 , 177 , 178 , 179 , 180 , 181 , 182 , 183 , 184 , 185 , 186 , 187 , 188 , 189 , 190 , 191 , 192 , 193 , 194 , 195 ], and 2 in Japanese [ 196 , 197 ]. The earliest included reference dated back to 1998 [ 153 ] (shown in Fig. 2 ), after which the remaining articles were distributed from 2000 to 2020 (45 articles in the 2000s, while 131 in the 2010s and 2 in the 2020s). The year 2012 witnessed the largest volume of publication (n = 26 articles, 14.53%). For funding sources or sponsors (Additional file 1 : Appendix S2), in addition to 57 articles not mentioning the funding/sponsor (hereinafter as non-funded articles), there were 116 articles (64.8% of the 179 articles) supported by 56 kinds of government funding from 12 countries/government organizations and, still, 9 articles (5.03%) by 10 kinds of industry/institute funding sources/sponsors from 4 countries (America, Australia, French and German). Among them, 3 articles [ 29 , 62 , 74 ] claimed to have been funded or sponsored by both government and industry. China had undertaken the most government/school-level funding projects (39 of 56 projects, 69.64%).
The publications (number of articles) on the safety of GM food by year
The periodicals that have published more than 5 included articles were Food and Chemical Toxicology (published 25 included articles), EFSA Journal (13), Regulatory Toxicology and Pharmacology (9), Journal of Hygiene Research (9) and Chinese Journal of Food Hygiene (8). 11 of 13 authors, who have published ten or more included studies, were from European Food Safety Authority and published 12 included articles as co-authors. They were Christina Tlustos (published 12 included articles), Claudia Bolognesi (12), Konrad Grob (12), Vittorio Silano (12), Andre Penninks (11), Gilles Riviere (11), Holger Zorn (11), Karl-Heinz Engel (11), Yi Liu (11), Natalia Kovalkovicova (10), Sirpa Karenlampi (10). In addition to the above 12 articles, the top 3 of the 11 authors who published five or more included studies was Yang Xiao-Guang (from Chinese Center for Disease Control and Prevention, published 11 included articles), Wang Jing (from Tianjin Centre for Disease Control and Prevention, published 10 included articles) and Zhuo Qin (from Chinese Center for Disease Control and Prevention, published 7 included articles). The top 5 affiliations which published included articles were Chinese Center for Disease Control and Prevention (published 16 included articles), Tianjin Centre for Disease Control and Prevention (12), European Food Safety Authority (12), National Chung Hsing University (10), International Rice Research Institute (9).
Of the 204 included studies, one was a double-blind crossover trial ( n = 36) in humans and the others were all animal studies. Individual sample sizes of the total 54,392 study population ranged from 4 (cats) [ 153 ] to 21,000 (Atlantic salmon) [ 23 ]. The studies involved 14 different kinds of animals (see Table 1 ). Apart from the most commonly used rats/mice (in 160 studies, 78.82%), pigs and chicks were two of the most extensively studied animals (in 23 studies, 11.33%). For themes of the 178 included animal studies, 158 were on clinical and 20 were on agricultural and animal husbandry. For the ones on clinical, 117 were on general toxicity (8 on acute, 6 sub-acute, 84 sub-chronic, 16 chronic toxicity, and still 3 on both acute, sub-acute and sub-chronic toxicity), 35 on specific toxicity (15 on reproductive and developmental toxicity, 16 on immunotoxicity, 3 on teratogenic effect and 1 on mutagenicity), 3 on allergenicity, 1 on learning and memory ability, 1 on athletic ability and 1 on both sub-chronic toxicity and allergenicity.
For interventions/exposures, 31 kinds of GM food were identified, including 18 kinds of GM plant food, 7 kinds of GM animal food and 6 kinds of GM microorganism food. Each included study covered one intervention/exposure, except for one study, Chen [ 29 ], that involved two kinds of GM products (sweet pepper and tomato) modified with the same gene (coat protein gene of cucumber mosaic virus), respectively, in two experimental groups. Maize, rice and soybean were the three most popular kinds of GM plant food (taken 79.38%) in research while milk/milk powder and animal-derived protein occupied the top two in GM animal food (56.25%). As for GM microorganism products, 5 kinds of food/feed enzyme derived from 5 different kinds of GM fungi or bacteria as well as 1 kind of microorganism-derived protein were among included studies.
Methodological quality of the animal studies
According to our predefined quality assessment criteria, all of the studies were identified as being unclear or having a high risk of bias (Fig. 3 ). None of the studies were reported to blind researchers from knowing which intervention each animal received. None of the studies reported prior sample-size calculation, 31 studies (15.27%) described wrong randomization procedures or did not mention the method of “randomization”, and 12 studies (5.91%) did not report adequate allocation concealment. 28 studies (13.79%) described that the groups were similar at baseline and 76 studies (37.44%) claimed that the housing conditions of animals from the various experimental groups were identical. 10 studies (4.93%) described randomly pick an animal during outcome assessment while 7 studies (3.45%) failed to select animals at random for outcome assessment. 88 studies (43.35%) completely used objective outcome indicators for outcome measurement. 185 studies (91.13%) reported consistent outcomes in the method and result sections while 5 studies did not, but none of the study protocols were available.
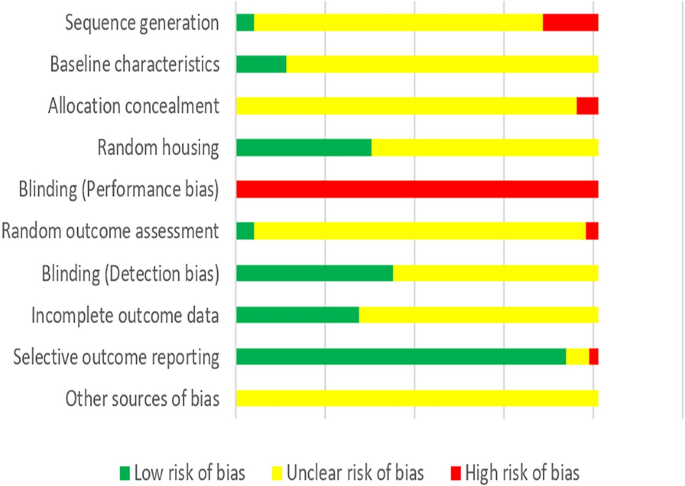
Risk of bias of the included animal studies
Incidence of adverse events/effects
No meta-analysis was conducted due to the significant heterogeneity of the primary studies. Among the 204 studies, a total of 29 studies (14.22%) from 23 articles reported 37 adverse events, involving 13 on mortality, 6 on reproductive toxicity, 3 on carcinogenesis and 15 on other biomarkers (including one human trial). It is worth noting that when, in one study, there were multiple aspects of adverse events on “other biomarkers”, we recorded it as 1 adverse event. Then, 22 serious adverse events (59.46% of adverse events) were identified in 16 studies (7.84% of the included studies and 55.17% of the studies reporting adverse events, marked in the tables with double asterisks). The SAEs mainly rested on mortality (13 studies), tumour or cancer (3), significant low in the number of pup deliveries (2), decreased learning and reaction abilities (1), severe stomach inflammation (1), intestinal adenoma lesions (1), and other pathology abnormalities (1) as hypertrophies and hyperplasia in mammary glands and pituitary, liver congestions and necrosis as well as severe chronic progressive nephropathies.
The incidence of adverse events reporting in government funding, industry funding and non-funded articles were 10.34% (12 of 116), 33.33% (3 of 9) and 15.79% (9 of 57), respectively. When comparing the adverse event reporting rates using the Chi-square test, we found that there were no significant differences either between industry funding and government funding ( χ 2 = 2.286, P = 0.131), industry funding and non-industry funding ( χ 2 = 1.761, P = 0.185) or funded and non-funded articles ( χ 2 = 0.491, P = 0.483).
Incidence of adverse events/effects in human trial
As for the human trial [ 97 ], shown in Table 2 , a randomized double-blind crossover design was conducted for acute consumption of two single breakfasts, with a 14-day washout period, containing either seed oil generated from transgenic Camelina sativa plants or commercially blended fish oil. 36 healthy people were randomly allocated into two groups and venous blood samples were collected after the postprandial session, 8 h after each meal. No follow-up was reported. No major adverse symptoms or health effects were reported but some unrelated minor illnesses for the 72 postprandial sessions from 36 participants, such as minor upper respiratory tract infections (2.78%), minor nose bleed (1.39%), pyelonephritis (1.39%) and headaches (8.33%).
Incidence of adverse events/effects in animal studies
For the 203 animal studies, 28 studies (13.79%) from 22 articles reported 36 adverse events, including 13 on mortality (Table 3 , 36.11%), 6 on reproductive toxicity ( Table 4 , 16.67%), 3 on carcinogenesis (Table 5 , 8.33%) and 14 on other biomarkers (Additional file 1 : Appendix S3, 38.89%).
All causes of death were included in this analysis and 11 of the 13 studies claimed that the mortality was not significantly different between the groups or had nothing to do with GM food. One study (Ermakova [ 37 ]) reported higher pup mortality in the Roundup-Ready soya (40.3.2 line) group compared with the controls. In Séralini [ 74 ] , the general cause of death was large mammary tumours in females and other organ problems in males. Besides, rats in the Roundup-tolerant GM NK603 maize groups were 2–3 times more likely to die than controls, and more rapidly.
With respect to effects on reproduction, 5 animal feeding studies were reported to trigger reproductive toxicity but one study (Cisterna [ 31 ]) claimed to have no substantial impact on fertility. The reproductive toxicity manifested in the significant low in the number of deliveries, survival rate (from birth to weaning), litter weight, litter size and weight of some organs in the pups. For example, in Ermakova I 2005, the rats fed with Roundup-Ready soya had a 55.6% pup mortality rate during lactation periods compared to 9% in the control of traditional soya and 6.8% in the reference group. The pups kept dying during the lactation period while pups from the control group only died during the first week. Cyran N 2008 a and Cyran N 2008 c [ 32 ] were two rat feeding studies reported in one article, both given NK603 × MON810 maize. A multi-generation study was conducted as Cyran N 2008 a while Cyran N 2008 c did a continuous breeding study. Both of them indicated that fewer sum of pups was born and weaned in the GM groups. Pup losses, in Cyran N 2008 a, overall generations were about twice as many pups lost as compared to the control group (14.59% vs 7.4%) but was not significantly different and significantly lower litter weight was also reported in Cyran N 2008 c.
Three mouse/rat feeding studies reported triggering cancers/tumours when Tang [ 156 ] attributed the incidence of the tumour to the elder age of rats. Séralini 2014 (on Roundup-tolerant GM maize) found that females in the treatment groups almost always developed large mammary tumours more often than and controls. As for males, 4 times larger palpable tumours than controls were presented which emerged up to 600 days earlier. Cyran 2008 b [ 32 ] revealed a life term study where mice in the three groups were fed with transgenic maize NK603xMON810 (from 33.0% in the diet), control isoline diet and GM-free Austrian corn reference diet, respectively. The survival rate was not significantly different while cancer (leucosis) was the common cause of death.
GM food-related adverse events
Among the 37 adverse events reported, 16 of them claimed to have nothing to do with GM food, while the rest 21 (from 17 studies) did not, still leaving the question open. The GM food-related adverse events existed in mortality (2 studies), reproductive toxicity (5), carcinogenesis (2), and other biomarkers (12).
By gathering evidence, we identified 3 kinds of GM food associated with adverse events, GM soybean, GM maize as well as GM rice. For the 17 studies involved in the GM food-related adverse events, 4 studies were absent of information on the GM event of their test substance and the remainder concentrated on 7 GM events (3 studies on NK603 × MON810 maize, 2 on GTS 40-3-2 soybean, 2 on NK603 maize, 2 on MON863 maize, 2 on MON810 maize, 1 on maize mixed with MON863 × MON810 × NK603, NK603 × MON810 and NK603 and 1 on GM Shanyou 63 rice). When searching in the GM Approval Database on the ISAAA website, we found that all of the first 6 GM events listed, all developed by Monsanto Company, had been on regulatory approval for food, feed and cultivation in multiple countries/regions, including the European Union. GM -39 Shanyou 63 was developed in China and given approval for food, feed, and cultivation only by China in 2009.
Summary of findings
We included 203 in vivo animal studies and 1 human trial, and all of the studies were identified as being unclear or having a high risk of bias. Overall, we reported two main findings. First, we identified 37 adverse events for GM food consumption while 22 of them (59.46%) were serious adverse events extracted from 16 animal studies (7.84%). SAEs were mortality, tumour or cancer, significantly low in the number of pup deliveries, decreased learning and reaction abilities, severe stomach inflammation, intestinal adenoma lesions, and other pathological abnormalities in the mammary glands, pituitary, liver and kidney.
Second, there were 21 GM food-related adverse events indicating that GM food may have effects on increased mortality (2 studies), reproductive toxicity (5 studies), which referred to significantly low fertility in parental generation and low survival rate, litter weight, litter size and weight of some organs in the pups, carcinogenesis (2 studies) and other biomarkers (12 studies). The effect-related GM food included 7 GM events (NK603 × MON810 maize, GTS 40-3-2 soybean, NK603 maize, MON863 maize, MON810 maize, MON863 × MON810 × NK603 maize and GM Shanyou 63 rice), which had all been on regulatory approval for food, feed and cultivation in some countries/regions.
Agreements and disagreements with other reviews
To our knowledge, there have been 3 previous systematic reviews (SRs) [ 198 , 199 , 200 ] and 6 conventional reviews [ 16 , 201 , 202 , 203 , 204 , 205 ] addressing similar research questions on the unexpected effects of GM food consumption. Keshani et al. [ 198 ], searching in 4 English databases, included experimental studies on GM crops’ potential effects on sperm parameters. The study finally included 7 rat feeding studies, which were all identified in our study, and indicated no harm to GM plants consumers. Edge et al. [ 199 ] addressed 30 review questions for including human studies, published in recent 20 years (1994–2014), on health effects of genetically engineered (GE) food crops, but found no human study on 25 questions. The remaining 5 questions, related to allergenicity and nutrient adequacy, were answered based on 21 human studies. The human studies were all excluded in our research because of no direct ingestion of GE food in the allergenicity assessment studies or no targeted outcomes in the nutrient assessment trial. To illustrate, the above-mentioned nutrient assessment clinical trial evaluated the effect of carrots containing twofold higher calcium content on calcium absorption and we thought it was not on outcome related to adverse events/effects. The conclusion of the research also supported that there were no clear adverse health effects associated with the consumption of GE food. Moreover, Dunn et al. [ 200 ] included both human and animal studies for examining the allergenicity of GM organisms and finally found 34 human studies and 49 animal studies eligible. In addition to 32 human studies which involved human serum for IgE binding or inhibition studies and not direct consumption of GM product, the rest 2 [ 206 , 207 ]studies were on actual ingestion of a GM food. However, they were not included in our research because of not targeted study type and unrelated outcomes. The conclusion agreed with the first two SRs that GM foods did not appear to be more allergenic than their conventional counterparts.
As for conventional reviews, Domingo showed special attention to the safety of GM food and published four literature reviews in 2000 [ 203 ], 2007 [ 204 ], 2011 [ 205 ] and 2016 [ 16 ]. Domingo searched two databases, PubMed and Scopus, to assess adverse/toxic effects of GM plants. In the latest updated review, he addressed the conclusion that GM soybeans, rice, corn/maize and wheat would be as safe as the parental species of these plants. However, our results may not be consistent with Domingo’s conclusion: we focus on a summarization of adverse events for GM food consumption through a systematic search in 7 databases; we identified 37 adverse events, 22 serious adverse events and 21 GM food-related adverse events; GM maize, soybean and rice with some specific GM events were all related to GM food-related adverse events. In addition, Domingo found a notable advance of studies published in scientific journals by biotechnology companies. Coincidentally, we did a Chi-square test to compare the adverse event reporting rates and found no significant differences between industry funding, government funding and non-funded articles. Besides, our systematic review validated Domingo’s findings that some GM plants were studied scarcely in recent years including GM potatoes discussed in the controversy of Pusztai case.
Strengths and limitations
In this review, a systematic search of major databases was conducted to identify all available studies in all languages on the adverse effects/events of GM food consumption. To make the inclusion and data synthesis comprehensive, both in vivo human and animal studies in all fields were included, with no limitations on the type of participant, type of intervention/exposure or whether control was included. The terms used for searching, containing all kinds of names of GM food, were based on a basic search on the internet by the researchers and the list was perfected as much as possible. With respect to additional searching, we went through multifarious news which reported controversy of GM food and thus we identified several hot studies by following the clue. In order to trace the potential conflicts of interest, we performed a Chi-square test for comparing adverse events report rates in articles funded by industry funding, government funding or unfunded articles, but found no statistical significance. Nevertheless, it was hard to conduct a quantitative data synthesis for the effects of GM food consumption on the adverse events because of the significant heterogeneity of the primary studies.
There are several limitations in this review. The methodological quality of the included studies is generally poor, which indicates a high or unclear risk of bias resulting from insufficient reporting of methodological components in the studies. Methodological quality may not be fully reflected based solely on the reporting of the manuscript. There were unclear descriptions of randomization procedures and a lack of blinding in all of the studies, which may have created potential performance biases and detection biases, as researchers might have been aware of the effects of interventions. The ability to perform meta-analysis was limited because of the heterogeneity of the participants, interventions (GM food in various GM events), comparisons, feeding doses, administration time, other exposure factors, and the variance of composite outcome measures used in the 204 included studies. When we did the manual search, we found that related publications were retracted sometimes, under the name of inadequate experimental designs or statistical analysis. For example, Séralini 2012 was retracted by Food and Chemical Toxicology , but subsequently republished in another journal [ 14 , 74 ]. This indicates that it was hard for us to find the original full-text papers of the retracted publications and articles provided by databases still have some unavoidable publication bias. The retraction on controversial researches may also cause the controversy for the public to doubt the reality of the studies published and to concern the safety of GM food. In addition, the lack of human studies is another key limitation of this research. As for the searching strategy, we did not include publication types as newspaper articles and comments. This was thought to be a limitation of this research because these sources may give us clues of related researches and can help us to do a manual search comprehensively. It is also an implication for future systematic reviews.

Implications for research
Future research should be conducted in humans, especially observational cohort studies. High-quality animal studies according to the ARRIVE reporting standard focusing on reproductive toxicity and carcinogenesis are still needed. Trials or studies should be registered prospectively, and be accessible. Furthermore, to address public concerns, future studies should focus on SAEs and GM food-related adverse events reported in this research such as NK603 maize, MON863 maize and MON810 maize. Meanwhile, some implications of findings still could be explored such as how GM food affects people’s eating habits, labelling of GM food and public choice. Some of the included studies conducted an intergenerational or multigenerational evaluation of the safety of GM food, but only two studies (Cyran N 2008 a and Cyran N 2008 c) in one article reported adverse events related to fertility. The differences in the results may be due to different interventions/exposures (GM food in certain GM events), laboratory animals, intervention/exposure time, experiment environment, etc. Therefore, it is necessary for subsequent studies to start with intergenerational or multigenerational research to verify the safety of GM food in terms of study design.
Serious adverse events accounted for 59.46% of the total 37 identified adverse events of GM consumption, which include: mortality, tumour or cancer, significantly lower number of pup deliveries, decreased learning and reaction abilities, and organ abnormalities in the stomach, intestinal adenoma, mammary glands, pituitary, liver and kidney. The interventions/exposures in the adverse event related studies emphasized on GM soybean, maize and rice in specific GM events. Animal studies occupy the lowest hierarchy of evidence, and there are flaws in study design and is not convincing enough. The evidence on the effect of GM consumption on humans is still insufficient. Further clinical trials and long-term cohort studies in human populations, especially on GM food-related adverse events and the corresponding GM events, are still warranted. It is better to prove the safety before they are approved for food consumption and it also suggests the necessity of labelling on GM food so that consumers can make their own choice.
Availability of data and materials
All data generated or analysed during this study are included in this published article.
Abbreviations
Genetically modified
Deoxyribonucleic acid
China National Knowledge Infrastructure
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
Serious adverse event
Camelina sativa Seed oil
Blended fish oil
Body weight
Systematic reviews
Genetically engineered
Hundleby PA, Harwood WA (2019) Impacts of the EU GMO regulatory framework for plant genome editing. Food Energy Secur. https://doi.org/10.1002/fes3.161
Article Google Scholar
Sprink T, Eriksson D, Schiemann J et al (2016) Regulatory hurdles for genome editing: process- vs. product-based approaches in different regulatory contexts. Plant Cell Rep 35:1493–1506. https://doi.org/10.1007/s00299-016-1990-2
Article CAS Google Scholar
Georges F, Ray H (2017) Genome editing of crops: a renewed opportunity for food security. GM Crops Food 8:1–12. https://doi.org/10.1080/21645698.2016.1270489
ISAAA (2018) Global status of commercialized biotech/GM Crops in 2018: biotech crops continue to help meet the challenges of increased population and climate change. ISAAA Brief No. 54 [Online]. http://www.isaaa.org/resources/publications/briefs/54/default.asp . Accessed 17 July 2020
Andrew J, Ismail NW, Djama M (2018) An overview of genetically modified crop governance, issues and challenges in Malaysia. J Sci Food Agric 98:12–17. https://doi.org/10.1002/jsfa.8666
Hickey LT, Hafeez AN, Robinson H et al (2019) Breeding crops to feed 10 billion. Nat Biotechnol 37(7):744–754. https://doi.org/10.1038/s41587-019-0152-9
Giraldo PA, Shinozuka H, Spangenberg GC et al (2019) Safety assessment of genetically modified feed: is there any difference from food? Front Plant Sci 10:1592. https://doi.org/10.3389/fpls.2019.01592
ISAAA (2016) Global status of commercialized biotech/GM crops: 2016. ISAAA Brief No. 52 [Online]. http://www.isaaa.org/resources/publications/briefs/52/default.asp . Accessed 17 July 2020
Pray C, Huang J, Hu R, Deng H et al (2018) Prospects for cultivation of genetically engineered food crops in China. Global Food Secur Agric Policy Econ Environ 16:133–137. https://doi.org/10.1016/j.gfs.2018.01.003
Martinelli L, Karbarz M, Siipi H (2013) Science, safety, and trust: the case of transgenic food. Croat Med J 54:91–96. https://doi.org/10.3325/cmj.2013.54.91
Klümper W, Qaim M (2014) A meta-analysis of the impacts of genetically modified crops. PLoS ONE. https://doi.org/10.1371/journal.pone.0111629
Losey JE, Rayor LS, Carter ME (1999) Transgenic pollen harms monarch larvae. Nature 399:214. https://doi.org/10.1038/20338
Ewen SW, Pusztai A (1999) Effect of diets containing genetically modified potatoes expressing Galanthus nivalis lectin on rat small intestine. Lancet 354:1353–1354. https://doi.org/10.1016/S0140-6736(98)05860-7
Séralini GE, Clair E, Mesnage R et al (2012) Long term toxicity of a Roundup herbicide and a Roundup-tolerant genetically modified maize [retracted in: Food Chem Toxicol. 2014 Jan;63:244]. Food Chem Toxicol 50(11):4221–4231. https://doi.org/10.1016/j.fct.2012.08.005
Genetic Literacy Project (2017) Where are GMOs grown and banned? [Online]. https://gmo.geneticliteracyproject.org/FAQ/where-are-gmos-grownand-banned/ . Accessed 5 Sept 2020
Domingo JL (2016) Safety assessment of GM plants: an updated review of the scientific literature. Food Chem Toxicol 95:12–18. https://doi.org/10.1016/j.fct.2016.06.013 ( Epub 2016 Jun 16 )
Moher D, Liberati A, Tetzlaff J et al (2009) Preferred reporting items for systematic reviews and meta analyses: the PRISMA statement. BMJ. https://doi.org/10.1136/bmj.b2535
FDA (2016) What is a serious adverse event? [Online]. https://www.fda.gov/safety/reporting-serious-problems-fda/what-serious-adverse-event . Accessed 28 Sept 2020
Al-Harbi A, Lary S, Edwards MG et al (2019) A proteomic-based approach to study underlying molecular responses of the small intestine of Wistar rats to genetically modified corn (MON810). Transgenic Res 28(5–6):479–498. https://doi.org/10.1007/s11248-019-00157-y
Appenzeller LM, Munley SM, Hoban D et al (2009) Subchronic feeding study of grain from herbicide-tolerant maize DP-Ø9814Ø-6 in Sprague-Dawley rats. Food Chem Toxicol 47:2269–2280. https://doi.org/10.1016/j.fct.2009.06.014
Bai H, Wang Z, Hu R et al (2015) A 90-day toxicology study of meat from genetically modified sheep overexpressing TLR4 in Sprague-Dawley rats. PLoS ONE. https://doi.org/10.1371/journal.pone.0121636
Bakke-Mckellep AM, Koppang EO, Gunnes G et al (2007) Histological, digestive, metabolic, hormonal and some immune factor responses in Atlantic salmon, Salmo salar L, fed genetically modified soybeans. J Fish Dis 30:65–79. https://doi.org/10.1111/j.1365-2761.2007.00782.x
Bakke-McKellep AM, Sanden M, Danieli A et al (2008) Atlantic salmon ( Salmo salar L.) parr fed genetically modified soybeans and maize: histological, digestive, metabolic, and immunological investigations. Res Vet Sci 84:395–408. https://doi.org/10.1016/j.rvsc.2007.06.008
Buzoianu SG, Walsh MC, Rea MC et al (2012) Effect of feeding genetically modified Bt MON810 maize to ∼ 40-day-old pigs for 110 days on growth and health indicators. Animal 6:1609–1619. https://doi.org/10.1017/S1751731112000249
Buzoianu SG, Walsh MC, Rea MC et al (2012) Effects of feeding Bt maize to sows during gestation and lactation on maternal and offspring immunity and fate of transgenic material. PLoS ONE. https://doi.org/10.1371/journal.pone.0047851
Carman JA, Vlieger HR, Ver Steeg LJ et al (2013) A long-term toxicology study on pigs fed a combined genetically modified (GM) soy and GM maize diet. J Organic Systems 1:1–12
Google Scholar
Chen X, Gao MQ, Liang D et al (2017) Safety assessment of genetically modified milk containing human beta-defensin-3 on rats by a 90-day feeding study. Food Chem Toxicol 100:34–41. https://doi.org/10.1016/j.fct.2016.12.012
Chen YN, Hwang WZ, Fang TJ et al (2011) The impact of transgenic papaya (TPY10-4) fruit supplementation on immune responses in ovalbumin-sensitised mice. J Sci Food Agric 91:539–546. https://doi.org/10.1002/jsfa.4218
Chen ZL, Gu H, Li Y et al (2003) Safety assessment for genetically modified sweet pepper and tomato. Toxicology 188:297–307. https://doi.org/10.1016/s0300-483x(03)00111-2
Chukwudebe A, Privalle L, Reed A et al (2012) Health and nutritional status of Wistar rats following subchronic exposure to CV127 soybeans. Food Chem Toxicol 50:956–971. https://doi.org/10.1016/j.fct.2011.11.034
Cisterna B, Flach F, Vecchio L et al (2008) Can a genetically-modified organism-containing diet influence embryo development? A preliminary study on pre-implantation mouse embryos. Eur J Histochem 52:263–267. https://doi.org/10.4081/1226
Cyran N, Gülly C, Handl S et al (2008) Biological effects of transgenic maize NK603xMON810 fed in long term reproduction studies in mice. FiBL, Wien
de Vendômois JS, Roullier F, Cellier D et al (2009) A comparison of the effects of three GM corn varieties on mammalian health. Int J Biol Sci 5:706–726. https://doi.org/10.7150/ijbs.5.706
Delaney B, Appenzeller LM, Munley SM et al (2008) Subchronic feeding study of high oleic acid soybeans (Event DP-3Ø5423-1) in Sprague-Dawley rats. Food Chem Toxicol 46:3808–3817. https://doi.org/10.1016/j.fct.2008.10.003
EFSA Panel on Genetically Modified Organisms (GMO), Naegeli H, Birch AN et al (2018) Assessment of genetically modified soybean MON 87751 for food and feed uses under Regulation (EC) No 1829/2003 (application EFSA-GMO-NL-2014-121). EFSA J 16(8):e05346. https://doi.org/10.2903/j.efsa.2018.5346
El-Shamei ZS, Gab-Alla AA, Shatta AA (2012) Histopathological changes in some organs of male rats fed on genetically modified corn (Ajeeb YG). Am J Sci 10(8):684–696
Ermakova I (2005) Influence of genetically modified soya on the birth-weight and survival of rat pups. In: Epigenetics, transgenic plants& risk assessment.
Gao MQ, Zhang R, Yang Y et al (2018) A subchronic feeding safety evaluation of transgenic milk containing human β-defensin 3 on reproductive system of C57BL/6J mouse. Food Chem Toxicol 115:198–204. https://doi.org/10.1016/j.fct.2018.03.007
Gu J, Krogdahl Å, Sissener NH et al (2013) Effects of oral Bt-maize (MON810) exposure on growth and health parameters in normal and sensitised Atlantic salmon, Salmo salar L. Br J Nutr 109:1408–1423. https://doi.org/10.1017/S000711451200325X
Guo QY, He LX, Zhu H et al (2015) Effects of 90-day feeding of transgenic maize BT799 on the reproductive system in male Wistar rats. Int J Environ Res Public Health 12:15309–15320. https://doi.org/10.3390/ijerph121214986
Hammond B, Dudek R, Lemen J et al (2004) Results of a 13 week safety assurance study with rats fed grain from glyphosate tolerant corn. Food Chem Toxicol 42:1003–1014. https://doi.org/10.1016/j.fct.2004.02.013
Hammond B, Lemen J, Dudek R et al (2006) Results of a 90-day safety assurance study with rats fed grain from corn rootworm-protected corn. Food Chem Toxicol 44:147–160. https://doi.org/10.1016/j.fct.2005.06.008
He XY, Tang MZ, Luo YB et al (2009) A 90-day toxicology study of transgenic lysine-rich maize grain (Y642) in Sprague-Dawley rats. Food Chem Toxicol 47:425–432. https://doi.org/10.1016/j.fct.2008.11.032
Healy C, Hammond B, Kirkpatrick J (2008) Results of a 13-week safety assurance study with rats fed grain from corn rootworm-protected, glyphosate-tolerant MON 88017 corn. Food Chem Toxicol 46:2517–2524. https://doi.org/10.1016/j.fct.2008.04.005
Ibrahim MA, Okasha EF (2016) Effect of genetically modified corn on the jejunal mucosa of adult male albino rat. Exp Toxicol Pathol 68:579–588. https://doi.org/10.1016/j.etp.2016.10.001
Kiliç A, Akay MT (2008) A three generation study with genetically modified Bt corn in rats: Biochemical and histopathological investigation. Food Chem Toxicol 46:1164–1170. https://doi.org/10.1016/j.fct.2007.11.016
Kiliçgün H, Gürsul C, Sunar M et al (2013) The comparative effects of genetically modified maize and conventional maize on rats. J Clin Anal Med 4:136–139
Lee NJ, Yang BC, Hwang JS et al (2010) Effects of cloned-cattle meat diet on reproductive parameters in pregnant rabbits. Food Chem Toxicol 48:871–876. https://doi.org/10.1016/j.fct.2009.12.025
Lee NJ, Yang BC, Im GS et al (2013) No long-term feeding toxicities on the health status in rats fed with cloned Korean native beef cattle (Hanwoo) meat. Toxicol Pathol 41:872–879. https://doi.org/10.1177/0192623312470762
Lin HT, Lee WC, Tsai YT et al (2016) Subchronic immunotoxicity assessment of genetically modified virus-resistant papaya in rats. J Agric Food Chem 64:5935–5940. https://doi.org/10.1021/acs.jafc.6b02242
Liu P, He X, Chen D et al (2012) A 90-day subchronic feeding study of genetically modified maize expressing Cry1Ac-M protein in Sprague-Dawley rats. Food Chem Toxicol 50:3215–3221. https://doi.org/10.1016/j.fct.2012.06.009
Liu Q, Yang W, Li M et al (2017) Effects of 60-week feeding diet containing Bt rice expressing the Cry1Ab protein on the offspring of inbred Wuzhishan Pigs fed the same diet. J Agric Food Chem 65:10300–10309. https://doi.org/10.1021/acs.jafc.7b04067
Liu S, Li CX, Feng XL et al (2013) Safety assessment of meat from transgenic cattle by 90-day feeding study in rats. Food Chem Toxicol 57:314–321. https://doi.org/10.1016/j.fct.2013.04.00
Liu S, Liu HB, Wang HL et al (2019) Evaluation of behavioral profiles in mice fed with milk supplemented diets derived from human lactoferrin gene-modified cows. Regul Toxicol Pharmacol 104:133–140. https://doi.org/10.1016/j.yrtph.2019.03.008
Liu Y, Zhang S, Zhou Q et al (2020) Subchronic feeding toxicity studies of drought-tolerant transgenic wheat MGX11-10 in Wistar Han RCC rats. Food Chem Toxicol 137:111129. https://doi.org/10.1016/j.fct.2020.111129
MacKenzie SA, Lamb I, Schmidt J et al (2007) Thirteen week feeding study with transgenic maize grain containing event DAS-Ø15Ø7-1 in Sprague-Dawley rats. Food Chem Toxicol 45:551–562. https://doi.org/10.1016/j.fct.2006.09.016
Malatesta M, Boraldi F, Annovi G et al (2008) A long-term study on female mice fed on a genetically modified soybean: effects on liver ageing. Histochem Cell Biol 130(5):967–977. https://doi.org/10.1007/s00418-008-0476-x
Malatesta M, Caporaloni C, Gavaudan S et al (2002) Ultrastructural morphometrical and immunocytochemical analyses of hepatocyte nuclei from mice fed on genetically modified soybean [published correction appears in Cell Struct Funct. 2002 Oct;27(5):399]. Cell Struct Funct 27(4):173–180. https://doi.org/10.1247/csf.27.173
Malatesta M, Caporaloni C, Rossi L et al (2002) Ultrastructural analysis of pancreatic acinar cells from mice fed on genetically modified soybean. J Anat 201(5):409–415. https://doi.org/10.1046/j.0021-8782.2002.00103.x
Mao J, Sun X, Cheng JH et al (2016) A 52-week safety study in cynomolgus macaques for genetically modified rice expressing Cry1Ab/1Ac protein. Food Chem Toxicol 95:1–11. https://doi.org/10.1016/j.fct.2016.06.015
Nouri-Ellouz O, Zeghal N, Makni S et al (2015) New food from a potato somatic hybrid: nutritional equivalence and safety assessment by a feeding study on rats. J Sci Food Agric 95(9):1911–1917. https://doi.org/10.1002/jsfa.6898
Oliva N, Florida Cueto-Reaño M, Trijatmiko KR et al (2020) Molecular characterization and safety assessment of biofortified provitamin A rice. Sci Rep 10(1):1376. https://doi.org/10.1038/s41598-020-57669-5
Papineni S, Golden RM, Thomas J (2017) The aryloxyalkanoate dioxygenase-12 (AAD-12) protein is not acutely toxic in mice[J]. Food Chem Toxicol 110:200–203. https://doi.org/10.1016/j.fct.2017.10.036
Papineni S, Murray JA, Ricardo E et al (2017) Evaluation of the safety of a genetically modified DAS-444Ø6-6 soybean meal and hulls in a 90-day dietary toxicity study in rats. Food Chem Toxicol 109(Pt 1):245–252. https://doi.org/10.1016/j.fct.2017.08.048
Papineni S, Passage JK, Ekmay RD et al (2018) Evaluation of 30% DAS-444Ø6-6 soybean meal in a subchronic rat toxicity study. Regul Toxicol Pharmacol 94:57–69. https://doi.org/10.1016/j.yrtph.2018.01.005
Poulsen M, Kroghsbo S, Schrøder M et al (2007) A 90-day safety study in Wistar rats fed genetically modified rice expressing snowdrop lectin Galanthus nivalis (GNA). Food Chem Toxicol 45(3):350–363. https://doi.org/10.1016/j.fct.2006.09.002
Qian ZY, Zhang SJ, Zhang L et al (2018) Subchronic toxicity study in rats evaluating genetically modified DAS-81419-2 soybean. Regul Toxicol Pharmacol 96:48–56. https://doi.org/10.1016/j.yrtph.2018.04.019
Qian ZY, Bultman J, Papineni S et al (2018) Safety evaluation of DAS-44406-6 soybeans in Wistar rats. Regul Toxicol Pharmacol 92:152–164. https://doi.org/10.1016/j.yrtph.2017.11.016
Tudisco R, Calabrò S, Cutrignelli MI et al (2015) Genetically modified soybean in a goat diet: Influence on kid performance. Small Rumin Res 126:67–74. https://doi.org/10.1016/j.smallrumres.2015.01.023
Richards HA, Han CT, Hopkins RG et al (2003) Safety assessment of recombinant green fluorescent protein orally administered to weaned rats. J Nutr 133(6):1909–1912. https://doi.org/10.1093/jn/133.6.1909
Sanden M, Ornsrud R, Sissener NH et al (2013) Cross-generational feeding of Bt ( Bacillus thuringiensis )-maize to zebrafish ( Danio rerio ) showed no adverse effects on the parental or offspring generations. Br J Nutr 110(12):2222–2233. https://doi.org/10.1017/S0007114513001748
Schrøder M, Poulsen M, Wilcks A et al (2007) A 90-day safety study of genetically modified rice expressing Cry1Ab protein ( Bacillus thuringiensis toxin) in Wistar rats. Food Chem Toxicol 45(3):339–349. https://doi.org/10.1016/j.fct.2006.09.001
Séralini GE, Cellier D, de Vendomois JS (2007) New analysis of a rat feeding study with a genetically modified maize reveals signs of hepatorenal toxicity. Arch Environ Contam Toxicol 52(4):596–602. https://doi.org/10.1007/s00244-006-0149-5
Séralini GE, Clair E, Mesnage R et al (2014) Republished study: long-term toxicity of a roundup herbicide and a roundup-tolerant genetically modified maize. Environ Sci Eur 26(1):14. https://doi.org/10.1186/s12302-014-0014-5
Sheng Y, Qi X, Liu Y et al (2014) Subchronic toxicity study in vivo and allergenicity study in vitro for genetically modified rice that expresses pharmaceutical protein (human serum albumin). Food Chem Toxicol 72:242–246. https://doi.org/10.1016/j.fct.2014.07.030
EFSA Panel on Food Contact Materials, Enzymes, Processing Aids (EFSA CEP Panel) et al (2019) Safety evaluation of the food enzyme endo-1,4-β-xylanase from a genetically modified Bacillus licheniformis (strain NZYM-CE). EFSA J 17(4):e05685. https://doi.org/10.2903/j.efsa.2019.5685 ( Published 2019 Apr 30 )
EFSA Panel on Food Contact Materials, Enzymes, Flavourings and Processing Aids (CEF) et al (2018) Safety evaluation of the food enzyme endo-1,4-β-xylanase from a genetically modified Bacillus subtilis (strain LMG S-24584). EFSA J 16(10):e05447. https://doi.org/10.2903/j.efsa.2018.5447 ( Published 2018 September 27 )
EFSA Panel on Food Contact Materials, Enzymes and Processing Aids (CEP), Silano V et al (2018) Safety evaluation of the food enzyme endo-1,4-β-xylanase from a genetically modified Aspergillus oryzae (strain NZYM-FA). EFSA J 16(11):e05480. https://doi.org/10.2903/j.efsa.2018.5480 ( Published 2018 Nov 16 )
EFSA Panel on Food Contact Materials, Enzymes and Processing Aids (CEP), Silano V et al (2018) Safety evaluation of the food enzyme endo-1,4-β-xylanase from a genetically modified Trichoderma reesei (strain DP-Nzd22). EFSA J 16(11):e05479. https://doi.org/10.2903/j.efsa.2018.5479 ( Published 2018 Nov 30 )
EFSA Panel on Food Contact Materials, Enzymes, Processing Aids (CEP) et al (2019) Safety evaluation of the food enzyme pullulanase from a genetically modified Bacillus licheniformis (strain DP-Dzp39). EFSA J 17(1):e05554. https://doi.org/10.2903/j.efsa.2019.5554 ( Published 2019 Jan 10 )
EFSA Panel on Food Contact Materials, Enzymes, Processing Aids (CEP) et al (2018) Safety evaluation of the food enzyme α-amylase from a genetically modified Aspergillus niger (strain NZYM-MC). EFSA J 16(10):e05451. https://doi.org/10.2903/j.efsa.2018.5451 ( Published 2018 Oct 31 )
EFSA Panel on Food Contact Materials, Enzymes, Flavourings and Processing Aids (CEF) et al (2018) Safety evaluation of the food enzyme alpha-amylase from a genetically modified Bacillus licheniformis (strain NZYM-AN). EFSA J 16(7):e05317. https://doi.org/10.2903/j.efsa.2018.5317 ( Published 2018 Jul 6 )
EFSA Panel on Food Contact Materials, Enzymes, Flavourings and Processing Aids (CEF) et al (2018) Safety evaluation of the food enzyme glucose oxidase from a genetically modified Aspergillus oryzae (strain NZYM-KP). EFSA J 16(7):e05319. https://doi.org/10.2903/j.efsa.2018.5319 ( Published 2018 Jul 6 )
EFSA Panel on Food Contact Materials, Enzymes, Flavourings and Processing Aids (CEF) et al (2018) Safety evaluation of the food enzyme endo-1,4-β-xylanase from a genetically modified Aspergillus niger (strain XEA). EFSA J 16(4):e05228. https://doi.org/10.2903/j.efsa.2018.5228 ( Published 2018 Apr 27 )
EFSA Panel on Food Contact Materials, Enzymes, Flavourings and Processing Aids (CEF) et al (2018) Safety evaluation of the food enzyme aqualysin 1 from a genetically modified Bacillus subtilis (strain LMGS 25520). EFSA J 16(5):e05170. https://doi.org/10.2903/j.efsa.2018.5170 ( Published 2018 May 2 )
EFSA Panel on Food Contact Materials, Enzymes, Flavourings and Processing Aids (CEF) et al (2018) Safety evaluation of the food enzyme xylanase from a genetically modified Bacillus subtilis strain TD160(229). EFSA J 16(1):e05008. https://doi.org/10.2903/j.efsa.2018.5008 ( Published 2018 Jan 22 )
EFSA Panel on Food Contact Materials, Enzymes, Flavourings and Processing Aids (CEF) et al (2018) Safety evaluation of food enzyme xylanase from a genetically modified Bacillus subtilis (strain LMG S-27588). EFSA J 16(5):e05169. https://doi.org/10.2903/j.efsa.2018.5169 ( Published 2018 May 2 )
Talyn B, Lemon R, Badoella M et al (2019) Roundup ®, but not roundup-ready ® corn, increases mortality of drosophila melanogaster. Toxics. https://doi.org/10.3390/toxics7030038
Tang M, Xie T, Cheng W et al (2012) A 90-day safety study of genetically modified rice expressing rhIGF-1 protein in C57BL/6J rats [published correction appears in Transgenic Res. 2012 Aug;21(4):927]. Transgenic Res 21(3):499–510. https://doi.org/10.1007/s11248-011-9550-6
Tang X, Han F, Zhao K et al (2012) A 90-day dietary toxicity study of genetically modified rice T1C–1 expressing Cry1C protein in Sprague Dawley rats. PLoS ONE 7(12):e52507. https://doi.org/10.1371/journal.pone.0052507
Teshima R, Watanabe T, Okunuki H et al (2002) Effect of subchronic feeding of genetically modified corn (CBH351) on immune system in BN rats and B10A mice. Shokuhin Eiseigaku Zasshi 43(5):273–279. https://doi.org/10.3358/shokueishi.43.273
Trabalza Marinucci M, Brandi G, Rondini C (2008) A three-year longitudinal study on the effects of a diet containing genetically modified Bt176 maize on the health status and performance of sheep. Livest Sci 2008(113):178–190
Walsh MC, Buzoianu SG, Gardiner GE et al (2011) Fate of transgenic DNA from orally administered Bt MON810 maize and effects on immune response and growth in pigs. PLoS ONE 6(11):e27177. https://doi.org/10.1371/journal.pone.0027177
Walsh MC, Buzoianu SG, Gardiner GE et al (2012) Effects of short-term feeding of Bt MON810 maize on growth performance, organ morphology and function in pigs. Br J Nutr 107(3):364–371. https://doi.org/10.1017/S0007114511003011
Walsh MC, Buzoianu SG, Rea MC et al (2012) Effects of feeding Bt MON810 maize to pigs for 110 days on peripheral immune response and digestive fate of the cry1Ab gene and truncated Bt toxin. PLoS ONE 7(5):e36141. https://doi.org/10.1371/journal.pone.0036141
Wang X, He X, Zou S et al (2016) A subchronic feeding study of dicamba-tolerant soybean with the dmo gene in Sprague-Dawley rats. Regul Toxicol Pharmacol 77:134–142. https://doi.org/10.1016/j.yrtph.2016.02.001
West AL, Miles EA, Lillycrop KA et al (2019) Postprandial incorporation of EPA and DHA from transgenic Camelina sativa oil into blood lipids is equivalent to that from fish oil in healthy humans. Br J Nutr 121(11):1235–1246. https://doi.org/10.1017/S0007114519000825
Wu Y, Xu Y, Du Y et al (2017) Dietary safety assessment of genetically modified rice EH rich in β-carotene. Regul Toxicol Pharmacol 88:66–71. https://doi.org/10.1016/j.yrtph.2017.05.019
Xiao GJ, Jiang SW, Qian LL et al (2016) A 90-day feeding study in rats to assess the safety of genetically engineered pork. PLoS ONE 11(11):e0165843. https://doi.org/10.1371/journal.pone.0165843
Xie Z, Zou S, Xu W et al (2018) No subchronic toxicity of multiple herbicide-resistant soybean FG72 in Sprague-Dawley rats by 90-days feeding study. Regul Toxicol Pharmacol 94:299–305. https://doi.org/10.1016/j.yrtph.2018.02.004
Yang L, Sun Y, Wang Y et al (2014) Effects of dietary transgenic poplar leaf pellets on performance and tissues in rabbits. J Sci Food Agric 94(6):1163–1167. https://doi.org/10.1002/jsfa.6388
Yen GC, Lin HT, Cheng YH et al (2011) Food safety evaluation of papaya fruits resistant to papaya ring spot virus. J Food Drug Anal 19(2):269–377
CAS Google Scholar
Yong L, Liu YM, Jia XD et al (2012) Subchronic toxicity study of GH transgenic carp. Food Chem Toxicol 50(11):3920–3926. https://doi.org/10.1016/j.fct.2012.07.064
Zeljenková D, Aláčová R, Ondrejková J et al (2016) One-year oral toxicity study on a genetically modified maize MON810 variety in Wistar Han RCC rats (EU 7th Framework Programme project GRACE). Arch Toxicol 90(10):2531–2562. https://doi.org/10.1007/s00204-016-1798-4
Zeljenková D, Ambrušová K, Bartušová M et al (2014) Ninety-day oral toxicity studies on two genetically modified maize MON810 varieties in Wistar Han RCC rats (EU 7th Framework Programme project GRACE). Arch Toxicol 88(12):2289–2314. https://doi.org/10.1007/s00204-014-1374-8
Zhou C, Wang JW, Huang KL et al (2011) A 90-day safety study in Sprague-Dawley rats fed milk powder containing recombinant human lactoferrin (rhLF) derived from transgenic cloned cattle. Drug Chem Toxicol 34(4):359–368. https://doi.org/10.3109/01480545.2010.542465
Zhou XH, Dong Y, Xiao X et al (2011) A 90-day toxicology study of high-amylose transgenic rice grain in Sprague-Dawley rats. Food Chem Toxicol 49:3112–3118. https://doi.org/10.1016/j.fct.2011.09.024
Zhu HJ, Chen Y, Li YH et al (2015) A 90 day safety assessment of genetically modified rice expressing Cry1Ab/1Ac protein using an aquatic animal model [published correction appears in J Agric Food Chem. 2015 Aug 26;63(33):7462]. J Agric Food Chem 63(14):3627–3633. https://doi.org/10.1021/jf5055547
Zhu Y, He X, Luo Y et al (2013) A 90-day feeding study of glyphosate-tolerant maize with the G2-aroA gene in Sprague-Dawley rats. Food Chem Toxicol 51:280–287. https://doi.org/10.1016/j.fct.2012.09.008
Zou S, Tang M, He X et al (2015) A 90-day subchronic study of rats fed lean pork from genetically modified pigs with muscle-specific expression of recombinant follistatin. Regul Toxicol Pharmacol 73(2):620–628. https://doi.org/10.1016/j.yrtph.2015.09.009
Dong SS, Zhang DN, Zhang ZH et al (2019) Ecotoxicological effects of transgenic mCry1Ac maize (BT799) on zebrafish. Ying Yong Sheng Tai Xue Bao 30(8):2845–2853. https://doi.org/10.13287/j.1001-9332.201908.031
Hu Y, Piao J, Yang X et al (2012) Nutritional components and sub-chronic toxicity of genetically modified rice expressing human lactoferrin. Wei Sheng Yan Jiu 41(1):6–12. https://doi.org/10.2166/wst.2012.090
Bai H (2015) Bio-Safety assessment of transgenic sheep overexpressing oTLR4. PhD Thesis, China Agricultural University, Beijing, China
Bao ZK (2016) Safety assessment of GH-Transgenic dairy goats. MS Thesis, Nanjing Agricultural University, Nanjing, China
Cao ZH (2014) Safety assessment of transgenic Bt rice in growing pig diet. MS Thesis, Henan University of Science and Technology, Henan, China
Chen XP, Zhuo Q, Piao JH et al (2004) Immunotoxicologic assessment of transgenetic rice. J Hyg Res 33(1):77–80
Chu HH, Si QQ, Xu Y et al (2016) Effect of genetically modified soybean meal on the immune function, intestinal digestive enzyme activities and serum biochemical indexes of growing pigs. J Qingdao Agric Univ Nat Sci 33(02):119–123. https://doi.org/10.3969/J.ISSN.1674-148X.2016.02.008
Dai YN, Yang XZ, Liu Y et al (2018) Acute oral toxicity and 90 days feeding test of recombinant human lactoferrin. J Hyg Res 47(02):286–311
Du HF (2006) Safety assessment of transgenic rice used in broiler diet. PhD Thesis, Chinese Academy of Agricultural Sciences, Beijing, China
Feng XL, Wang HL, Li CX et al (2017) Safety assessment of meat from transgenic cattle by 90-day feeding study in rats. J Hyg Res 29(1):19–25. https://doi.org/10.13590/j.cjfh.2017.01.005
Feng YQ, Hu J, Zhi Y et al (2013) The effect of exposure to transgenic Bt rice on the immune system of parental female rats. Chin J Food Hyg 25(4):298–302
Feng YQ, Wang EH, Zhi Y et al (2013) The effect of exposure to transgenic Bt rice on reproductive system of male offspring rats. Chin J Food Hyg 25(02):113–117
Guo MF (2018) A three generation study to evaluate reproductive and neurodevelopmental toxicity of genetically modified maize with Cry1Ab and epsps genes in SD rats. MS Thesis, Chinese Center for Disease Control and Prevention, Beijing, China
Hu J (2013) Establish of a rat model for development immunotoxicity and its application in safety evaluation of transgenic CryAb/Ac Rice. MS Thesis, Chinese Center for Disease Control and Prevention, Beijing, China
Huang Q, Xu HB, Gao F et al (2009) Anaphylactic reactions in WZS minipig orally induced by glycinin. J Hyg Res 38(05):531–534
Jia XD, Li N, Wang W et al (2005) Assessment of allergenicity of genetically modified rice S86 by BN rat model. Chin J Food Hyg 01:7–9. https://doi.org/10.13590/j.cjfh.2005.01.003
Li M (2012) Safety evaluation of recombinant herbicide-resistant protein AROa-CC-M and transgenic insect-resistant maize BT-799. MS Thesis, Chinese Academy of Agricultural Sciences, Beijing, China
Li M, Piao JH, Yang XG (2010) Subchronic toxicity test of genetically modified rice with double antisense starch-branching enzyme gene. J Hyg Res 39(04):436–443
Li R, Wang J, Jiang WL et al (2012) Effects of rice genetically modified with HJC-1 and G6-EPSPS genes on immunological parameters in Wuzhishan minipigs. J Environ Health 29(08):689–692. https://doi.org/10.16241/j.cnki.1001-5914.2012.08.017
Li YH, Piao JH, Chen XP et al (2004) Immunotoxicologic assessment on transgenic rice. China Public Health 20(04):20–22
Li YH, Piao JH, Zhuo Q et al (2004) Study on the teratogenicity effects of genetically modified rice with Xa21 on rats. J Hyg Res 33(06):710–712
Liang LQ, Wang J, Cao XC et al (2010) Toxicity analysis of common carp transferred salmon growth hormone gene. Food Sci 31(05):261–265
Liu HT, Wang CR, Liu L et al (2018) Acute toxicity of fresh leaves of insect resistant transgenic populus nigra to mice. J Anhui Agric Sci 46(01):94–136. https://doi.org/10.13989/j.cnki.0517-6611.2018.01.028
Liu HL, Wang J, Zeng Q et al (2012) Effects of rapeseed genetically modified with bar gene on immunological indicators in WZS minipigs. J Environ Health 29(11):977–980. https://doi.org/10.16241/j.cnki.1001-5914.2012.11.020
Liu SS, Tan JZ, Sun Z et al (2011) Effects of glyphosate—resistant soybean meal on immune function of AA broilers. Chin J Anim Sci 47(13):41–46
Liu S, Wang XD, Feng XL et al (2013) Twenty-eight days feeding study on human lactoferrin expressed by cattle mammary bioreactor in mice. Chin J Public Health 29(02):230–232
Liu YH, Jiang SQ, Zhang J et al (2018) Subchronic toxicity of genetically modified Herbicide-resistant maize MON87427 with Cp4epsps gene in Wistar rats. J Public Health Prevent Med 29(06):17–20. https://doi.org/10.3969/j.issn.1006-2483.2018.06.004
Liu YF, Liu WH, He L et al (2008) Acute toxicity and mutagenic effect of transgenic rice on mice. J Hunan Univ Sci Technol Nat Sci Ed 23(04):111–117
Liu YF, Liu WH, He L et al (2008) Effects of resistant insects transgenic hybrid rice 21S/MSB on behavior and physiology of SD rats. Life Sci Res 12(03):257–261. https://doi.org/10.16605/j.cnki.1007-7847.2008.03.013
Lu MJ, Li F, Zhou GL et al (2008) Assessment of an anti-LeETR1 genetically modified tomato on reproductive-development toxicity and transferability of the transgene. J Toxicol 22(04):272–274. https://doi.org/10.16421/j.cnki.1002-3127.2008.04.006
Lu CB, Lin ZB, Zhang Y et al (2016) Effects of glyphosate-resistant transgenic soybean on physical enginery of male mice. Acta Agriculturae Zhejiangensis 28(07):1115–1120. https://doi.org/10.3969/j.issn.1004-1524.2016.07.04
Lu CB, Yang DY, Gao Z et al (2012) Safety assessment of reproductive system in male mice fed with genetically modified soybeans. J Yangzhou Univ Agric Life Sci Ed 33(01):23–27. https://doi.org/10.16872/j.cnki.1671-4652.2012.01.006
Lu CB, Zhang Y, Chen BH et al (2017) Effects of glyphosate-resistant transgenic soybean on in vitro fertilization of male mice with reproductive damage. Acta Agriculturae Zhejiangensis 29(06):910–916. https://doi.org/10.3969/j.issn.1004-1524.2017.06.08
Lu CB, Zhou W, Liu B et al (2013) Effects of transgenic soybean on reproductive system in male mice. Soybean Sci 32(01):119–123
Lv L, Guo J, Li SF et al (2013) Effects of transphytase gene maize on organ development and pathological changes of broilers. Chin J Anim Sci 49(05):31–34
Ma BT, Yuan XY, Wang XD et al (2017) Animal experiment of recombinant human lactoferrin based on the 28 days repeated oral toxicity. J Hyg Res 46(03):443–454
Ma YM, Wang J, Jiang WL et al (2012) Subchronic oral toxicity of genetically modified cottonseed with FBP7-iaaM gene in rats. J Environ Health 29(11):1001–1007. https://doi.org/10.16241/j.cnki.1001-5914.2012.11.024
Qi XZ, Wang J, Zhou C et al (2010) Effect of transferred human lactoferrin milk powder on serum iron and ferritin in rats. Food Sci 31(23):340–343
Qin HF (2012) Safety assessment of rice genetically modified with Cry1Ac and sck feeding studies on broilers. PhD Thesis, Chinese Academy of Agricultural Sciences, Beijing, China.
Qiu ZL, Sun N, Wang J et al (2011) Sub-Chronic toxicity study of transgenic cottonseed in SD rats. Progr Mod Biomed 11(12):2215–2220. https://doi.org/10.13241/j.cnki.pmb.2011.12.010
Song LS, Gao GQ, Wei ZY et al (2017) Effects of Fat1 transgenic milk on the health and reproductive ability of mice. Lab Anim Sci 34(03):28–37
Song Y (2013) Establishment of immunotoxicity screening system in rodents and its application in immunotoxicity evaluation of pesticides and genetically modified foods. PhD Thesis, Chinese Center for Disease Control and Prevention, Beijing, China
Sun XW, Liang LQ, Yan XC et al (1998) Research on transgenic carp as food. High Technol Lett 03:3–5
Sun Z, Liu SS, Tan JZ et al (2011) Effects of glyphosate—resistant soybean meal on immune function of AA broilers. Chin J Anim Sci 23:836–841
Tan JZ (2011) The feed safety assessment of Glyphosate-Tolerant soybean meal in broilers. MS Thesis, Chinese Academy of Agricultural Sciences, Beijing, China
Tang XQ, Wang YF, Pei LJ et al (2019) Long-term toxicity study on transgenic rice T2A–1 with cry2A* gene. Chin J Food Hyg 31(6):510–516. https://doi.org/10.13590/j.jfh.2019.06.002
Tao R (2008) Detection of transgenic ingredients in feed and primary assessment on the safety of aquatic livestock fed transgenic soybean. MS Thesis, Chinese Academy of Sciences, Shandong, China
Wang EH (2014) A study to access effects of transgenic Cry1Ab/Ac Rice TT51 on reproductive and neural development in rats. PhD Thesis, Chinese Center for Disease Control and Prevention, Beijing, China
Wang EH, Yu Z, Fang HQ et al (2013) Effect of transgenic Bt rice TT51 on early physiological and neurological development of rats offspring. Chin J Food Hyg 25(06):485–488. https://doi.org/10.13590/j.cjfh.2013.06.008
Wang HM, Yin JY, Zhai WS, et al (2015) Toxic pathology of cynomolgus monkeys fed transgenic rice for 52 weeks. In: The 7th National Toxicology Conference of China Toxicology Society and the 8th Hubei Science and Technology Forum, Wuhan, China, pp 442–443
Wang J, Jiang WL, Wang XJ et al (2002) Toxicological safety evaluation of transgenic T5 line pepper. Chin J Urban Rural Ind Hyg 01:46
Wang J, Jiang WL, Wang Y et al (2012) Subacute oral toxicity of recombinant human lactoferrin from transgenic cows in rats on 28d. J Toxicol 26(05):393–397
Wang J, Li R, Liu HL (2012) Assessment of allergenicity of rice genetically modified with HJC-1 and G6-EPSPS genes by BN rats model. J Environ Health 29(11):967–970. https://doi.org/10.16241/j.cnki.1001-5914.2012.11.014
Wang J, Liu HL, Zeng Q et al (2012) Subacute toxicity of rapeseed genetically modified with bar gene in WZS minipigs. J Environ Health 29(11):980–984. https://doi.org/10.16241/j.cnki.1001-5914.2012.11.021
Wang J, Zhou C, Che HL et al (2010) Study on sub-chronic toxicity of powered milk containing transgenic lactoferrin on SD rats. Progr Mod Biomed 10(15):2809–2813. https://doi.org/10.13241/j.cnki.pmb.2010.15.002
Wang RY (2017) The effect of genetically modified feed on structure of mice testes. Livestock Poult Ind 28(06):6–7. https://doi.org/10.19567/j.cnki.1008-0414.2017.06.004
Wang R, Hu YC, Li M et al (2017) Study on the subchronic toxicity of transgenic DBN9978 herbicide resistant maize to rats. Food Nutr China 23(06):12–17
Wang XJ, Wang J, Liu HL et al (2012) Subchronic toxicity test of rice containing transgenic HJC-1 and G6-EPSPS in rats. J Environ Health 29(11):970–976. https://doi.org/10.16241/j.cnki.1001-5914.2012.11.019
Wang Y (2011) Food safety assessment of genetically modified milk with human lactoferrin gene. MS Thesis, Tianjin Medical University, Tianjin, China
Wang Y, Lai WQ, Chen JG et al (2000) Toxicity of anti-herbicide gene (BAR) transgenic rice. J Hyg Res 29(03):141–142
Wu JH (2013) The nutritional, edible safety and efficacy assessment of genetically modified rice with human lactoferrin gene and its purified protein and the nutritional assessment of genetically modified wheat expressing GmDREB/TaDREB4 genes with drought-resistance. Ph.D. Thesis, Chinese Center for Disease Control and Prevention, Beijing, China
Wu P, Su YL, Zhang J et al (2003) Safety evaluation of transgenic tomato against Cucumber Mosaic Virus. J Capital Univ Med Sci 24(03):254–258
Xu YJ (2012) The forage safety assessment of genetically modified organism corn to weaning piglets. M.S. Thesis, Fujian Agriculture and Forestry University, Fujian, China
Yu T, Liu Y, Wang JW et al (2017) Teratogenic test of recombinant human lactoferrin in rats. J Toxicol 31(03):247–250
Yuan JQ (2015) The detection of the transgenic GTS40-3-2 related genes and their products of livestock products sold and toxicology study of Sprague-Dawley rats ( Rattus norvegicus ). Ph.D. Thesis, Shanxi Agricultural University, Shanxi, China
Zhang L, Cheng C, He N et al (2011) Study on subchronic toxicity of transgenic soybean with high oleic acid on rats. J Toxicol 25(05):391–394. https://doi.org/10.16421/j.cnki.1002-3127.2011.05.012
Zhang L, Wang J, Jiang SQ et al (2016) Subchronic toxicity of genetically modified corn with Cry1Ab/Cry2Aj and G10evo (EPSPS) genes in rats. J Environ Health 33(07):585–589
Zhang LL (2018) The Unintended Effects of Long-Term Intergenerational Feeding Transgenic Maize Diets to Pure Line White Leghorn Chickens on the Intestinal Health. M.S. Thesis, Chinese Academy of Agricultural Sciences, Beijing, China
Zhang M, Zhuo Q, Tian Y et al (2012) Study on chronic toxicity of genetically modified rice expressing human lactoferrin. Chin J Food Hyg 24(06):391–394. https://doi.org/10.13590/j.cjfh.2012.06.008
Zhang Q (2014) Production of GH transgenic goat by somatic cell nuclear transfer. Ph.D. Thesis, Nanjing Agricultural University, Nanjing, China
Zhang WP, Li YY, Wang WG et al (2009) Detection of mutagenicity of chitinase and -1, 3 dextran gene in maize. Chin Remed Clin 9(05):405–407
Zhang ZY, Liu LJ, Zhang L et al (2010) Subchronic toxicity of Bt transgenic rice to mice. J Toxicol 24(02):126–129. https://doi.org/10.16421/j.cnki.1002-3127.2010.02.016
Zhao L, Zhang L, Zhang YY et al (2009) Immunotoxicological evaluation of transgenic soybean oil. China Health Care Nutr 11:5
Zhi Y, Liu HB, Di GY et al (2013) Genetic toxicity of transgenic human α-lactalbumin powdered milk. Carcinogen Teratogen Mutagen 25(02):124–133. https://doi.org/10.3969/j.issn.1004-616x.2013.02.010
Zhi Y, Liu HB, Di GY et al (2011) Study on sub-chronic toxicity of powered milk containing transgenic human α-lactalbumin. J Hyg Res 40(04):426–430. https://doi.org/10.19813/j.cnki.weishengyanjiu.2011.04.004
Zhong F (2013) Subchronic feeding study of transgenic BADH alfalfa on rabbits. M.S. Thesis, Shandong Agricultural University, Shandong, China
Zhou GL, Lu MJ, Chen YX et al (2007) Antisense LeETR1 transgenic tomato rats were fed for 4 weeks. J Toxicol 21(02):160–161
Zhou H (2012) Safety aeeseement of the phytase transgenic corn in the broiler diet. M.S. Thesis, Fujian Agriculture and Forestry University, Fujian, China
Zhou LG (2009) Research of high lysine transgenic paddy on broiler feed security. M.S. Thesis, Yangzhou University, Jiangsu, China
Zhou WL, Yang XF, Ping A et al (2014) Effects of transgenic Dwarf-mosaic-resistant maize on learning and memory abilities of rats. J Shanxi Agric Univ Nat Sci Ed 34(02):129–131. https://doi.org/10.13842/j.cnki.issn1671-8151.2014.02.006
Zhou XH (2012) Toxicological studies on the food safety of two transgenic rice. Ph.D. Thesis, Jiangsu University, Jiangsu, China
Zhou ZW, Wang DZ, Shen H et al (2012) Comprehensive evaluation on functions & safety of imported GM soybean using BDI-GS system. Soybean Sci 31(05):822–826
Zhu H, Zhu LY, Guo JY et al (2014) Safety evaluation of BT-799 corn on Wistar rats. Food Nutr China 20(09):63–67
Zhuo Q, Chen XP, Piao JH et al (2004) Experimental study on converting cowpea trypsin inhibitor rice for 90 days. J Hyg Res 33(02):176–179
Zhuo Q, Chen XP, Piao JH et al (2004) Study on teratogenic effect of cowpea trypsin inhibitor rice. J Hyg Res 33(01):74–77
Sakamoto Y, Tada Y, Fukumori N et al (2008) A 104-week feeding study of genetically modified soybeans in F344 rats. Shokuhin Eiseigaku Zasshi 49(4):272–282. https://doi.org/10.3358/shokueishi.49.272
Sakamoto Y, Tada Y, Fukumori N et al (2007) A 52-week feeding study of genetically modified soybeans in F344 rats. Shokuhin Eiseigaku Zasshi 48(3):41–50. https://doi.org/10.3358/shokueishi.48.41
Keshani P, Sharifi MH, Heydari MR et al (2020) The effect of genetically modified food on infertility indices: a systematic review study. Sci World J. https://doi.org/10.1155/2020/1424789
Edge MS, Kunkel ME, Schmidt J et al (2018) Evidence analysis library systematic review on advanced technology in food production. J Acad Nutr Diet 118(6):1106–1127. https://doi.org/10.1016/j.jand.2017.08.005
Dunn SE, Vicini JL, Glenn KC et al (2017) The allergenicity of genetically modified foods from genetically engineered crops: a narrative and systematic review. Ann Allergy Asthma Immunol 119(3):214–222. https://doi.org/10.1016/j.anai.2017.07.010
de Vos CJ, Swanenburg M (2018) Health effects of feeding genetically modified (GM) crops to livestock animals: a review. Food Chem Toxicol 117:3–12. https://doi.org/10.1016/j.fct.2017.08.031
Ricroch AE, Boisron A, Kuntz M (2014) Looking back at safety assessment of GM food/feed: an exhaustive review of 90-day animal feeding studies. Int J Biotechnology 13(4):230–256
Domingo Roig JL, Gómez Arnáiz M (2000) Riesgos sobre la salud de los alimentos modificados genéticamente: una revisión bibliográfica [Health risks of genetically modified foods: a literature review]. Revista espanola de salud publica 74(3):255–261
Domingo JL (2007) Toxicity studies of genetically modified plants: a review of the published literature. Crit Rev Food Sci Nutr 47(8):721–733. https://doi.org/10.1080/10408390601177670
Domingo JL, Giné Bordonaba J (2011) A literature review on the safety assessment of genetically modified plants. Environ Int 37(4):734–742. https://doi.org/10.1016/j.envint.2011.01.003
Teshima R, Watanabe T, Okunuki H et al (2002) Effect of subchronic feeding of genetically modified corn (CBH351) on immune system in BN rats and B10A mice. J Food Hyg Soc Jpn 43:273–279
Dubois AEJ, Pagliarani G, Brouwer RM et al (2015) First successful reduction of clinical allergenicity of food by genetic modification: Mal d 1-silenced apples cause fewer allergy symptoms than the wild-type cultivar. Allergy 70:1406–1412
Download references
Acknowledgements
We appreciate Yi-Zhen Li for participating in screening the titles and abstracts .
This work was supported by Innovation Team and Talents Cultivation Program of National Administration of Traditional Chinese Medicine (ZYYCXTD-C-202006). Prof. Nicola Robinson (visiting professor of Beijing University of Chinese Medicine) was funded by the International development and capacity enhancement of evidence-based Chinese medicine Project, Ministry of Science and Technology of the People's Republic of China, G20200001187.
Author information
Authors and affiliations.
Centre for Evidence-Based Chinese Medicine, Beijing University of Chinese Medicine, 11 Bei San Huan Dong Lu, Chaoyang District, Beijing, 100029, China
Chen Shen, Xiao-Wen Zhang, Wen-Bin Hou, Min Fang, Xun Li, Yu-Tong Fei, Nicola Robinson & Jian-Ping Liu
Beijing Institute of Radiation Medicine, Beijing, China
Xiang-Chang Yin
School of Chinese Medicine, Beijing University of Chinese Medicine, Beijing, China
Bo-Yang Jiao
Beijing Key Laboratory of the Innovative Development of Functional Staple and the Nutritional Intervention for Chronic Disease, China National Research Institute of Food & Fermentation Industries Co,. Ltd, Beijing, China
School of Life Sciences, Beijing University of Chinese Medicine, Beijing, China
School of Chinese Materia Medica, Beijing University of Chinese Medicine, Beijing, China
Xue-Hao Cheng & Jian-Xin Ren
Ruikang Hospital, Guangxi University of Chinese Medicine, Guangxi, China
School of Health and Social Care, London South Bank University, London, SE1 OAA, UK
Nicola Robinson
Institute for Excellence in Evidence-Based Chinese Medicine, Beijing University of Chinese Medicine, Beijing, China
Jian-Ping Liu
You can also search for this author in PubMed Google Scholar
Contributions
All work was done by the authors. JPL and YTF conceived the study and revised the manuscript. CS contributed to data searching, screening and extraction, analysis of the data, drafted and revised the paper and approved the final version to be submitted. XCY, BYJ, JP, XHC, JXR, JL, XWZ, HDL, WBH and MF participated in identifying or screening the titles, abstracts and full-text screening and data extraction. XL, NR and JPL advised on the analysis of the data and revised the manuscript. All authors read and approved the final manuscript.
Corresponding author
Correspondence to Jian-Ping Liu .
Ethics declarations
Ethics approval and consent to participate.
Not applicable.
Consent for publication
Competing interests.
The authors declared that the research was conducted in the absence of any commercial or financial relationships that could be constructed as a potential conflict of interest.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: appendix s1..
Search strategy applied in English language databases. Appendix S2. Funding sources or sponsors. Appendix S3. Adverse events/effects—other biomarkers.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Shen, C., Yin, XC., Jiao, BY. et al. Evaluation of adverse effects/events of genetically modified food consumption: a systematic review of animal and human studies. Environ Sci Eur 34 , 8 (2022). https://doi.org/10.1186/s12302-021-00578-9
Download citation
Received : 07 July 2021
Accepted : 11 December 2021
Published : 13 January 2022
DOI : https://doi.org/10.1186/s12302-021-00578-9
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Adverse event
- Genetically modified food
- Evidence-based medicine
- Systematic review
- Open access
- Published: 18 October 2012
The application of GMOs in agriculture and in food production for a better nutrition: two different scientific points of view
- M. Buiatti 1 ,
- P. Christou 2 &
- G. Pastore 3
Genes & Nutrition volume 8 , pages 255–270 ( 2013 ) Cite this article
63k Accesses
63 Citations
17 Altmetric
Metrics details
This commentary is a face-to-face debate between two almost opposite positions regarding the application of genetic engineering in agriculture and food production. Seven questions on the potential benefits of the application of genetic engineering in agriculture and on the potentially adverse impacts on the environment and human health were posed to two scientists: one who is sceptical about the use of GMOs in Agriculture, and one who views GMOs as an important tool for quantitatively and qualitatively improving food production.
Since the mid-1990s, the release of GMOs into the environment and the marketing of foods derived from GM crops has resulted in a scientific and public debate. Despite the potential benefits of the application of genetic engineering in agriculture in order to improve the quality and the reliability of the food supply, since the beginning, public and scientific concerns have been raised in many parts of the world about environmental and food safety of GM crops.
Two major different points of view have been expressed by the community: on one hand, recombinant DNA technology is seen as a potent tool for enhancing crop productivity (first generation GMOs) and food quality (second generation GMOs) or “drug factories”, for the production of vaccines and/or therapeutic medicines (third generation GMOs). GMO supporters point to evidence that GMOs must be considered essential for promoting sustainable agriculture, as they may be able to reduce agriculture’s environmental footprint, reducing the use of pesticides, saving fossil fuels, decreasing CO 2 emissions and conserving soil and moisture (James 2011 ). Supporters also consider GM crops indispensable in facing the severe global food and nutrition security problem in developing countries: although GM crops are not presented as the “absolute solution”, it has been stated that they could undoubtedly make a significant contribution to an array of measurements and incentives to this constantly growing problem (Conner et al. 2003 ).
On the other hand, antagonists argued that the side effects in terms of potentially adverse impacts on the environment and human health are still largely unknown, and probably unknowable for decades, and encourage waiting for the final outcome of further research and utilization. Many concerns have been raised for the environment: the capability of a GMO to escape from confinement and therefore potentially to transfer engineered genes into wild populations, the persistence of the gene after a GMO has been harvested, the susceptibility of non-target organisms to the gene product, the instability of new genes, the reduction of the spectrum of other plants resulting in a significant loss of biodiversity and an increase in the use of chemicals in agriculture. As for human health, the main concerns have been the possibility of a transfer of allergens into the new foods, the gene transfer from GM foods to human cells or to bacteria in the gastrointestinal tract, which can cause worries especially transferred genetic material proved to adversely affect human health the transfer of genes from GM plants into conventional crops or related species in the wild, as well as the mixing of GM crops with those derived from conventional seeds, that could have an indirect effect on food safety and food security.
The same debate also occurs at the societal level. It is obvious that without “approval” by society at large, GM crops will surely fail in the marketplace. The forthcoming years, then, will be crucial for the commercial and economically viable application of GMOs in agriculture and food production (Nap et al. 2003 ). Consumer attitudes with respect to genetically modified foods differ widely, particularly between North America and Europe. Information asymmetry, incomplete information and uncertainty arise as a result of concerns over GMOs. The major concerns have arisen particularly in western Europe, where a general lack of awareness about how our food production system evolved, the strong opposition by activist groups and a steady stream of negative opinions in the media rapidly increased the resistance to GMO production and use among consumers. This point of view was rapidly endorsed by politicians. The European consumer confidence in the safety of food supplies had decreased significantly in the 1990s as a result of a number of food “scandals” that, although not related to GM foods, left consumers to a state of uncertainty regarding the validity of risk assessments, both with regard to consumer health and environmental risks, focusing in particular on long-term effects.
With regard to foods derived from GM crops, consumers have not perceived any direct advantage, and therefore the public attention focused on the risk side of the risk–benefit equation.
As a consequence of the different American and European public attitudes towards this technology and the foods produced, the regulatory approaches in Europe and North America are essentially different: in the EU regulatory policy is based on the process of making GM crops; in the USA on the characteristic of the GM product.
In the EU, strong public concerns about GMOs triggered the imposition in October 1998 of a de facto moratorium on the authorization of new releases of GMOs in the European Union, and even stricter standards were proposed in the EU’s revised Directive 90/220 of August 2000. Before the imposition of the moratorium , releases of GMOs were reviewed on a case-by-case basis and had to be approved at every step from laboratory testing through field testing to final marketing. By contrast, the permit procedure in the United States is far simpler and faster.
Consumer concerns have triggered a discussion on the desirability of labelling GM foods, allowing an informed choice. The different attitudes of the consumers in EU and USA have led to marked differences in national labelling requirements. The US Food and Drug Administration does not require labelling of GM foods per se, but only if the transgenic food is substantially different from its conventional counterpart. The EU, by contrast, requires labelling of all foodstuffs, additives and flavours containing 1 % or more genetically modified material (Regulations 1139/98 and 49/2000).
Within this picture, seven different questions were posed to two scientists representing the two different points of view: Prof. Marcello Buiatti (Dept of Genetics at the University of Florence, Italy), who is sceptical about the use of GMOs in agriculture, and Prof. Paul Christou (Dept. of Plant Production and Forestery Sciences, University of Lleida, Spain), who represents those who view GMOs as an important tool for quantitatively and qualitatively improving food production.
Concerns have been raised that GM crops will hybridize with related species resulting in the introgression of transgenes to weedy relatives. For transgenes conferring resistance to pests, diseases and herbicides, it has been suggested that this can also lead to an enhanced fitness, survival and spread of weeds. On the other hand, GM crops have been proposed as “friendly” bioherbicides and bioinsecticides, suggesting that future GMOs will be useful for soil, water, and energy conservation and for the natural waste management. Are GMOs, then, a risk or an opportunity to maintain the health of the environment?
M. Buiatti:
I do not really see at this moment any possible advantage from GMO cultivation for the health of the environment. I do not really remember reductions in tillage practices favorable to the environment, as the only reduced practice is man-made weed destruction, certainly advantageous for the owners of the fields because of the very low level of manpower needed in the case of herbicide resistant crops, but of irrelevant as far as environment management is concerned. Moreover, as the herbicide can in this case be utilized all along the cycle, many more treatments can be carried out and it is widely known that glyphosate exerts detrimental effects on the soil ecosystem and may be polluting ground water.
On the other hand, transgene flow to weedy relatives particularly of canola, an outbreeding species liable to hybridize to other Brassicaceae (Beckie et al. 2009 ), and of maize in the area of the origin of the species, has been shown to occur (see for instance Snow 2009 ). However, only the maize case is relevant for the ecosystem structure, as it may hybridize with the ancestor species teosinte, while the real danger of the hybridization of weed Brassica species is mainly relevant for agriculture as it may render them resistant to herbicides. The problem here is that gene flow evaluations are based on pollution probability studies, forgetting the fact that even low levels of pollen can flow to a few unintended GM plants can lead to each producing a large amount of pollen, putatively polluting neighbouring plants. Anyway the problem about pollution is not in my opinion health related but property related, as leading companies can sue any owner of a field having, without his will, even very few GM plants, according to the industrial patents covering all fields containing any amount of the patented objects.
Much more relevant are, in my opinion, the side-effects on ecosystems and particularly on the soil animal and microbial flora, both extremely relevant factors for the life of the highly inter-connected agro-ecosystem. On the contrary, as already discussed by Ch. Darwin in his treatise on worms, plants are connected through reciprocal exchange of nutritional components with the microbial flora and fauna, both liable to be affected by all agricultural practices from the use of chemicals, soil management, water distribution, etc. For this reason, the impact of GMOs will not only derive from the plant itself but also from its exudates and the agricultural practices to which single genetically modified plants (GMPs) are connected. In the case of GMPs resistant to herbicides, for instance, the effects of the herbicide itself (particularly glyphosate and the adjuvants present with it in commercial preparations) should be considered in all details in a holistic way, as summarized in an excellent review by Huber 2010 , a very good example of risk evaluation through the integrated analysis of all the interactions with the plant itself, the nutrients in the soil and the soil microflora (on that subect, see also the review by Kremer and Means 2009 ). As far as the Bt GMPs are concerned, as thoroughly discussed in a recent review by Icoz and Stotzky 2008 , Bt plants showed changes in the microbial communities' compositions, both as far as bacteria and fungi are concerned, and particularly mycorrhizae, a key group of fungi for plants nutrition (Castaldini et al. 2005 ; Giovannetti et al. 2005 ). All the examples just quoted are, however, only the direct effects on the agro-ecosystem of the transgenic plants themselves or of the agricultural practices associated to their cultivation but, as I will try to better discuss in my answer to a different question, the real damage to the ecosystem is not directly related to the genetic modification but derives from the economy, particularly of soybean cultivation in very large areas of developing countries and also of emerging ones, particularly in Latin America where large forested or traditionally cultivated areas have been converted to industrial soybean cultivation with a very relevant loss of the pre-existing biodiversity.
I shall not comment on the possible future GMPs not on the market, as I am used, particularly in this area, not to try to predict the future behaviour of industry and the markets, the reason being discussed in my response to question 3. In fact, the presently utilized technology of genetic engineering has not been improving for a long time; the research intensity of leading GM Companies has been constantly reduced. Particularly, no innovation has been introduced to avoid “unintended effects” of the interactions between the inserted sequences and the receiving organism, nor has any technique been developed allowing one to aim the construct in specific areas of the host genome to avoid the insertion and negative modification of relevant sequences of the original DNA. Certainly the lack of progress in those fields may seem amazing and can be justified only with the fact that the revenues of the leading companies do not come from innovation but from the royalties of already existing GMPs, advertising and stock exchange speculation.
P. Christou:
Gene flow does occur between GM crops and related weeds and wild species, but the consequences of this process are exaggerated. Taking herbicide tolerance first, it is important to recognize that although herbicide-tolerant transgenic plants have a selective advantage in cultivated areas where herbicides are applied, they have no such advantage elsewhere. Therefore the energetic burden of producing unnecessary detoxification enzymes and the genetic burden of possessing inefficient herbicide target enzymes can often make such plants less fit than their weedy and wild counterparts, naturally selecting against them in wild ecosystems where herbicides are not used, or in rotational agricultural ecosystems where the herbicide is rotated (Gressel 2002 ). Weedy species also tend to be more resistant to insects and diseases than domesticated crops because they produce toxins that fend off pests and pathogens. These toxins have been bred out of our crops because the toxins affect humans, too, which is one reason crops are more susceptible than weeds to insect pests (Gressel 2008 ). Therefore, additional resistance transgenes have little impact on the fitness of weeds and are soon diluted from the population (Gressel 2008 ). In cases where a real risk is envisaged, such as controlling weedy rice in monoculture rice paddies, there are adequate technologies to mitigate gene flow (Gressel 2012 ). Different species (transgenic or otherwise) will undergo different levels of gene flow, so the only rational way forward is to evaluate them on a case-by-case basis using science-based risk assessment procedures clearly divorced from any political interference. The risk assessment must be initiated by the applicants developing GM crops, and they must supply all necessary information to the regulatory agencies appointed to perform such evaluations professionally and impartially (EFSA 2010 ). Notwithstanding the above, the fear of gene flow damaging the environment has resulted in European legislation to mitigate gene flow using a plethora of barrier and distance-related measures (Ramessar et al. 2010 ; Morris and Spillane 2010 ). Molecular biologists have also been encouraged to develop strategies to prevent gene flow by developing systems for selectable marker excision (Hare and Chua 2002 ). Ironically, the focus on gene flow means that little is being done to prevent or control the introduction of exotic and potentially invasive species, which in principle could be far more damaging than new varieties (including GM varieties) of the domesticated plant species currently under cultivation. A 10-year study in the UK demonstrated that GM corn, potato, rapeseed and sugar beet lines are no more invasive or persistent than their conventional counterparts (Crawley et al. 2001 ).
GM crops are currently submitted for risk assessment on a case-by-case basis using science-based risk assessment procedures, and it is acknowledged that (as with all other technologies and, indeed, in all other areas of life) we cannot expect zero risk. Some modifications could be irreversible and the question is then whether it might be prudent to accept a “very low risk” of “irreversible hazardous modifications”, or follow the “zero risk approach” as contemplated by a number of environmental organizations. It might therefore be instructive to address this theoretical scenario. The reversibility of the GM trait is influenced by the competitive advantage under natural conditions conferred by the introduced trait and the ability of the GM plant to transfer such traits to wild plants. Neither of these risk factors has been found in the GM crops cultivated in the EU or any of the crops that have received a positive EFSA Scientific Opinion. The target of “zero risk” to the environment as enshrined in the current EU legislation for GMOs would be sound if agriculture in its entirety were a “zero risk” activity for humans, but this is not the case. However, the approval delays in the EU do pose a definitive and quantifiable risk for the safety of humans and the environment as they contribute to the perpetuation of older and less safe technologies, such as the use of chemical pesticides.
The focus on risks also draws attention away from the clear environmental benefits of GM crops, including the fact that herbicide-tolerant crops allow the adoption of reduced tillage and conservation tillage practices, increasing carbon retention in the organic matter of the soil, restoring populations of organisms living or nesting in the soil, e.g. earthworms, ants and birds (Tebrügge 2010 ; Belmonte 1993 ), and reducing the use of fuel needed for tillage operations (Service 2007 ; Brookes and Barfoot 2009 ). Similarly, pest-resistant GM crops expressing Bt proteins are environmentally beneficial because there is no need to spray broad-spectrum pesticides onto the plants, thus reducing the use of fuel and avoiding environmental contamination with chemical pollutants (Smale et al. 2009 ), a strategy that also benefits non Bt-corn growers (Hutchinson et al. 2010 ). Bt toxins are highly specific and are confined within the plant so that only pests actually attacking plants are affected, not beneficial insects and microbes. Bt toxins are therefore recommended for more sustainable integrated pest control programs (Romeis et al. 2006 ; Sanahuja et al. 2011 ). It is again ironic that detractors focus on the theoretical risks of gene flow from pest-resistant crops (theoretical because Bt-crops have a 100 % safety record in the 15 years since they were first planted commercially (Sanahuja et al. 2011 )) while ignoring the much greater environmental burden of broad-spectrum insecticides that essentially wipe out the entire insect ecosystem in an agricultural setting and are well known to be toxic to humans (Sanahuja et al. 2011 ). The impressive safety record of Bt crops is unprecedented, yet Bt crops in Europe are subject to draconian rules which even the EC has admitted make no sense (Ramessar et al. 2008a , 2009 ; Sanahuja et al. 2011 ).
Soil animal and microbial flora are very important factors for the agro-ecosystem, so it is important to ask whether GM crops have a negative effect on soil organisms. All peer-reviewed studies published thus far clearly demonstrate that any effect of GM crops on soil microbial flora is lower in magnitude than effects related to location, seasonal variations and (most importantly) conventional/organic agricultural practices such as tillage. EFSA concluded in its Opinion for continued cultivation of MON810 in the EU ( The EFSA Journal 2009 1149, 1–85): “The EFSA GMO Panel is of the opinion that potential effects on soil microorganisms and microbial communities due to corn MON810 if they occur, will be transient, minor and localised in different field settings and are likely to be within the range currently caused by other agronomic and environmental factors.” This conclusion on the safety of MON810 corn has been confirmed in recent papers on the impact of Bt corn on endophytic bacteria (Prischl et al. 2012 ). The impact of the herbicide glyphosate on NK603 corn mycorrhiza has also been found to be lower than conventional herbicides (Barriuso et al. 2010 , 2011a ), and does not change the corn rhizobacterial communities compared to those in untreated soil (Barriuso et al. 2011b ).
The introduction of foreign genes into food plants has been considered to have an unexpected and negative impact on human health, in particular for the introduction of new allergens and/or for the effects of possible horizontal gene flow or any other unknown and uncontrollable effect of the transferred gene. On the other hand, future GM organisms are likely to include plants with increased nutrient levels, plants producing pharmaceutically important molecules and plants with improved resistance to diseases, cold, or drought, thus suitable for increasing food security in disadvantaged areas. Are GMOs, then, a risk or a potential benefit for human health?
GM food crops were first planted commercially in 1996 and in 2010 they were cultivated on 148 million ha of land (James 2010 ). In all that time there has been not one single report of an adverse event caused by the consumption of GM food products; no reports of toxicity or allergenicity. Indeed, no difference in nutritional or organoleptic properties compared to the non-GM equivalent [have been reported] at all. Several widely discussed reports about the potential adverse effects of GM crops in animal studies have also been comprehensively debunked (Sears et al. 2001 ; Shelton and Sears 2001 ; Ricroch et al. 2010 ; Batista and Oliveira 2009 ). StarLink is often put forward as an example of potential toxicity or allergenicity, but it is important to note that the summary of the investigation by the US Centers for Disease Control is very clear: “These findings do not provide any evidence that the reactions that the affected people experienced were associated with hypersensitivity to the Cry9c protein.” The details can be found at http://www.cdc.gov/nceh/ehhe/Cry9Creport/ .
More recently, microRNAs from plants were reported to accumulate in mammalian blood and tissues, where they “might be able to regulate gene expression” ( http://the-scientist.com/2011/09/20/plant-rnas-found-in-mammals/ ). The subtitle of this publication, “MicroRNAs from plants accumulate in mammalian blood and tissues”, is grossly exaggerated. The biological activity was not seen in a normal diet, but after ingestion of a raw rice diet by rats equivalent to 33 kg of rice per day for a human . The report also neglects to mention that microRNAs are a natural form of gene regulation in all plants and animals, and that humans therefore consume millions of plant and animal miRNAs every day in normal diets without any known effect. Furthermore, the pharmaceutical industry has struggled for over a decade to develop oral medications based on RNA-mediated gene regulation without success, because it is extremely difficult to persuade the human body to absorb these molecules in a functional form because of the significant degradation that takes place in the gut. It is interesting that reaction to this report has immediately focused on the potential for negative effects while leaving out an important potential application of microRNAs: “Although the team has still a long way to go in elucidating the mechanisms by which plant microRNAs can regulate gene expression in humans, these initial results promise to increase the understanding of how specific ingredients in food can mediate health and disease”. This was a statement by Clay Marsh, Director of the Center for Personalized Health Care at the Ohio State University College of Medicine, who studies microRNA expression in human blood but was not actually involved in the research discussed above.
Despite the extraordinary safety record of GM crops, GM agriculture as a whole faces the most restrictive regulatory framework outside the nuclear industry (Ramessar et al. 2008a , 2009 , 2010 ). This dogmatic requirement for “zero risk” is astonishing when one considers that all other technologies and activities in the human sphere of existence, including nuclear energy, are considered as part of a risk/benefit trade-off. For example, all known drugs have adverse effects but are accepted because they have a beneficial role in treating disease, many (natural) foods have well-known adverse health effects yet people consume them anyway, and other allergenic plant-derived products are accepted without question—for example, approximately 5 % of the world’s population are allergic to natural rubber but there is no crusade to have this substance banned and the plantations destroyed (Sussman et al. 1991 ). The central issue with GM crops is that because there are no concrete adverse effects for people to quantify, they can only focus on theoretical and largely unquantifiable ones. The hysteria about horizontal gene transfer is a key example of this phenomenon (Twyman et al. 2009 ). It is well known that genes can be transferred horizontally between bacteria, and from bacteria to higher plants (one of the methods scientists use to transfer DNA to plants exploits bacteria). There is no evidence that antibiotic resistance transgenes have transferred horizontally from plants to bacteria that are human pathogens, therefore placing human health at risk, but no scientist can claim such an event is impossible, so there has to be a small but non-zero theoretical risk (in the same way that there is a small but non-zero theoretical risk that someone walking down the street may be struck by a piano falling from a cargo plane). However, on the basis of infinitesimal theoretical risk, the use of antibiotic resistance genes as markers in GM plants is now strongly discouraged (Ramessar et al. 2007 ). The great irony is that these antibiotic resistance genes are themselves entirely natural and are present in billions of bacteria all over the world. Every time someone eats non-GM fruits and vegetables, they are consuming these bacteria and the genes they contain. As stated above, gene transfer between bacteria is a well-known and very common natural occurrence so, again theoretically, these natural bacteria would provide a much more likely source of antibiotic resistance to transfer to human pathogens in the gut, yet this process has never been documented (Ramessar et al. 2007 ). Finally, the selective antibiotics are no longer used in a clinical setting, so even if resistance did jump to human pathogens, it would have no impact at the point of care. Even so, millions of euros were invested into the development of politically expedient technologies to remove antibiotic resistance markers, thus ensuring the risk of transference from GM plants was reduced from almost zero to zero, when nature teems with the very same antibiotic resistance genes and no steps are taken to avoid them . There are no other technologies that demand zero risk, certainly none with such impressive credentials that the EU could state in a report following a 15-year study (1985–2000) involving 400 public research institutions and costing 70 million euros: “… genetically modified plants and products derived from them present no risk to human health or the environment……these crops and products are even safer than plants and products generated through conventional processes” (EC Research 2001 ; Kessler and Economidis 2001 ). In a subsequent report covering the next decade, the EU commission affirmed this outcome and reiterated: “The main conclusion to be drawn from the efforts of more than 130 research projects, covering a period of more than 25 years of research, and involving more than 500 independent research groups, is that biotechnology, and in particular GMOs, are not per se more risky than e.g. conventional plant breeding technologies” (European Commission 2010a ).
As with the first question, the focus on imperceptible risks means that the many potential benefits of GM agriculture are ignored. It is generally acknowledged that first-generation GM crops provide higher yields with fewer inputs (principally fuel and pesticides), which has important economic benefits for the agricultural industry in the industrialized world, but the more significant positive effects are seen in the developing world where GM crops allow subsistence farmers not only to survive but to take surplus produce to market, providing additional wealth that supports education, improves access to medicines, and leads to the empowerment of women (Christou and Twyman 2004 ; Yuan et al. 2011 ). However, the hysterical anti-GM activism and the resulting political expediency is seriously delaying this process, particularly by holding back the deployment of newer first-generation GM crops that are protected from drought, salinity and better suited to grow in hostile environments, as well as second-generation GM crops that have enhanced output traits such as better nutritional composition (Farre et al. 2011b ). The industrialized world has the luxury of choice, at least for the time being, but in the developing world GM crops could turn the tide against plant diseases and pests, eliminate damaging agricultural practices, reduce hunger and malnutrition and produce cheap medicines in response to some of the world’s most pressing socioeconomic concerns (Farre et al. 2010 ; Gómez-Galera et al. 2010 ). It is no exaggeration to say that the anti-GM precedent currently set by Europe is indirectly contributing to death on a massive scale in Africa and Asia (Potrykus 2010 ).
When I am asked this question I usually answer that I do not know for sure and the reason for this answer is that control agencies are not reliable. I do not frankly know exactly what happens in all countries, but I believe it to be similar to what we have here in Europe with EFSA, which I do know fairly well. The main problems with EFSA are two. In the first place, EFSA does not utilize independent laboratories for the control of GMO bio-safety, and therefore relies on the answers from the producer companies to the questions posed by specific scientific committees. Therefore, while those committees are, as far as we know, quite independent, of course companies certainly are not and, moreover, they keep sending back the conclusions of their laboratories and not the raw data. Therefore it is also impossible to check the reliability of the statistical treatment of the results, as happened in the unfortunate case of the Maize MON863. In that case the producing company (Monsanto) was obliged by a German Court to release the data, and I personally saw the amazingly poor statistical treatment utilized. In the second place, EFSA guidelines do not take into account the rapidly improving tools for risk assessment and do not carry out what is called “whole cycle analysis”, looking at all possible direct and indirect effects of GMOs not only on human health, but also on environment and agriculture as has been rightly done in the present questionnaire. So, at the molecular level whole genome analyses putatively leading to “unintended effects” are never carried out and old fashioned Southern’s are readily accepted in their place, proving the presence and integrity of the engineered construct but not the putative presence of other DNA fragments scattered into the receiving genome as found in many cases, for instance, by Svitashev and Somers ( 2001 ) and many others. This omission does not allow the screening of putative changes in host gene expression, the transcription of fusion RNAs and proteins, etc. (see Rosati et al. 2008 ). Moreover, epigenomic analyses are not requested, studies on the metabolomes and physiological changes, particularly in hormone patterns, the study of effects on the environment are limited to the possible weed resistance to herbicides, and so on. Finally, requested studies of GMO toxicity in rats are very poor and carried out for periods that are much too short. However, my feeling (not my scientific opinion due to the lack of data) is that health risks of transgenic food on the market now are limited, micro-RNAs may in theory block genes having complementary sequences. I think that the risks from blocks in genes relevant to human health is very low, but it may happen. It should be recalled from this point of view that not-aimed insertion of DNA into the receiving genomes is the main reason of the unfortunate failure of gene therapy in humans. The real danger being, also in this case, glyphosate and its adjuvants. As far as the future is concerned, I am really extremely worried about open air cultivation of plants which are transgenic for pharmaceuticals, because in that case cross-pollination with vaccines or other proteins could be really dangerous, as it could lead to unneeded pharmaceuticals in food. Of course, on the other hand, plants transgenic of proteins not liable to be produced by prokaryotes, if the plants are not to be grown in open air, may certainly be interesting.
When judging a novel technology the first question to be answered is whether the technology is really innovative and successful.
Which are the major technical advancements in plant genetic engineering since the release into the market of the first genetically modified products?
The first GM crops on the market were engineered for herbicide tolerance; these were soon followed by plants engineered for pest resistance. More than 15 years later, almost all commercially approved GM crops still have one or both of these traits, and for the first time in 2009–2010, plants stacked with multiple traits were grown more widely than those with single traits. Indeed, July 2009 saw the commercial release of the most ‘stacked’ GM crop thus far, i.e. Smartstax corn, jointly developed by Monsanto and Dow AgroSciences, combining eight different herbicide and pest resistance traits.
Despite the rather limited scope of current commercial GM crops, the development pipeline is incredibly rich and diverse. Innovations in the development of GM crops fall into four major areas, which can be described as improved first-generation crops (focusing on input traits but using innovative approaches), novel second-generation crops (delivering better output traits), third-generation crops (delivering value added products) and technical developments such as the control of transgene expression (Farre et al. 2011a ; Bai et al. 2011 ).
In the first category, several new approaches have been developed to achieve pest resistance in addition to the current reliance on Bt genes, because Bt genes do not exist to counter the effects of all known pests. Also, there is the potential for pest populations to evolve resistance to single Bt toxins (Christou et al. 2006 ; Ferry et al. 2006 ). As well, there are alternative protein toxins such as lectins that work against recalcitrant sap-sucking insects; novel approaches include the expression of toxin fusions (Mehlo et al. 2005 ) and the use of RNA interference by targeting genes essential for insect development (Huvenne and Smagghe 2010 ). A small number of commercial crops are resistant to diseases, such as virus-resistant papaya, squash, plum and bean plants, and rice plants resistant to bacterial infections. Many additional GM crops resistant to various viral, bacterial and fungal diseases are under development, using a vast number of different approaches, such as enhancing natural plant defenses, the expression of pathogen proteins, the expression of plant-proteins that repel specific pathogens and even the expression of mammalian antibodies that neutralize pathogens inside the plant (Collinge et al. 2010 ). Many concepts have also been developed that will help crops withstand harsh environments, especially drought, high levels of salinity, waterlogging and poor soil quality (Cominelli and Tonelli 2010 ).
Although first-generation crops benefit farmers mainly by allowing them to overcome biological and environmental extremes (biotic and abiotic stresses), the next breakthrough in GM agriculture will be the deployment of second-generation crops, where the benefits are targeted at consumers. The key examples here are Golden Rice, which produces enough β-carotene in the polished grain to ensure that consumers relying on a cereal diet do not suffer vitamin A deficiency (Potrykus 2010 ), and the multivitamin corn and high zeaxanthin corn produced in our laboratory (Naqvi et al. 2009 , 2011a , b ; Zhu et al. 2008 ). In multivitamin corn, three distinct metabolic pathways are modified to simultaneously enhance the levels of three key vitamins. The rapid progress of nutritionally enhanced GM crops through the development pipeline will save millions of lives and reduce the impact of malnutrition in the world’s poorest areas (Zhu et al. 2007 ).
There has also been remarkable progress in the development of third-generation GM crops, which are not intended for human consumption but instead have valuable industrial uses (Naqvi et al. 2011a ; Ramessar et al. 2008c ). At the forefront are pharmaceutical crops producing proteins or small-molecules of medical relevance (Ma et al. 2003 , 2005 ; Ramessar et al. 2008c ). In our laboratory we have achieved the production of an HIV-neutralizing antibody in corn which could be used as a microbicide component to help prevent the spread of the virus (Ramessar et al. 2008b ). The value of producing such molecules in plants rather than mammalian cells or bacteria as is usually the case is the reduced costs, the better safety profile (no human or animal pathogens, no endotoxins) and the massive production scale that can be achieved with little additional effort (Stoger et al. 2005 ). Also in this category are plants used to produce industrial raw materials (e.g. starch, rubber) and plants used to produce fuel (e.g. bioethanol, biodiesel). In both cases, it is important to avoid competition with food crops.
Finally, a variety of novel technologies have been developed to control transgene expression, e.g. spatiotemporal and inducible promoters (Peremarti et al. 2010 ), and to increase the precision of transgene integration into plants, e.g. transcriptional activator-like effector nucleases (TALENs) and zinc-finger nucleases (Weinthal et al. 2010 ).
Some might argue that the development pipeline discussed above is misleading because only four cultivated crops with the same two modifications have reached the market. Does this then mean that all the others have been failures? I would say that the answer is emphatically no. First, there are other products on the market that do not receive as much attention, e.g. virus-resistant rainbow papayas that have been consumed in the US for years and that have recently been approved in Japan, one of the most stringent markets. GM sweet corn is also approved for human consumption in the US and GM beans are now grown in Brazil. The problem with the adoption of novel GM crops is the huge cost of regulatory approvals (industry estimates suggest each new crop will cost $US 100 million in development). This means that only major staple crops currently offer any hope of investors recovering their R&D costs. Although the EU claims to defend a “knowledge-based bioeconomy”, some patents covering glyphosate-tolerant sugar beet expired before the cultivation of this crop was approved in Europe. On top of this, the activity of NGOs that oppose GM crops is often supported by public administrations and welcomed by the media, resulting in approved GM crops like Bt potato being rejected by the food industry to avoid campaigns against their brands. We can define them as technical and regulatory successes, but marketing failures, as happens in many other areas of the economy.
Golden Rice will soon be grown on a large scale in the Philippines. It has taken years to obtain regulatory approval and funding for this was raised only recently. Because Golden Rice does not directly benefit farmers, there was no incentive for industry to cover the approval costs. My opinion is that these costs should have been covered by government public health authorities, as they stand to lose the most from a population riven by vitamin A deficiency and they have the most to gain from the health benefits derived from this crop.
After the development by M.D.Chilton in 1991 of the first method of plant genetic engineering through the usage of Agrobacterium tumefaciens, the first transgenic plant (tobacco), was produced in 1983 and a few years later Bt genes for resistance to insects and genes for the resistance to herbicides were introduced into crops. The first transgenic cultivar to enter the market was the Tomato Flavr Savr, resistant to rotting, in 1994, but it was very soon withdrawn, because of unexpected negative side effects of the transformation. In 1996 both insect resistant maize and RR soybean herbicide resistant plants were introduced into the market. As reported by Clive James in the annual review of cultivated GMPs in 2010, only four cultivated crop plants, still bearing the same two modifications, are in the market and have been widely commercialised (soybean, maize, cotton, canola). Therefore no new products have been released in the market with success, in spite of the many announced GMPs, and a few have been withdrawn from the market like the first one, the tomato Flavr Savr and the last one as far as I know, the so-called “Golden rice”, of which a new cultivar producing more pro-vitamin A than the former is expected but has not been released. In the meantime, research intensity on the part of the leading companies has been decreasing as discussed by Schimmelpfennig et al. ( 2004 ). Obviously, this speaks very little for an innovative technology whose first products have been on the market for almost 15 years. The scientific reasons for these failures lie in the complexity of the plant system and the consequent “unintended effects” deriving from the aforementioned interactions between the inserted construct and the host plant. Of course this does not mean that new useful and efficient products could not be obtained, but this can occur only if new, reliable methods of control of the dynamics of the plant system are developed. Apparently and unfortunately, the leading companies do not seem interested in following this process, probably because, as discussed further in the answer to question 6, incomes of leading companies derive from the control of the market, the intellectual property rights of the commercialised products, the stock exchange etc. and not from innovations in the field.
The import of affordable GM soybeans and GM corn from Brazil, USA, Argentina and other countries is pivotal in maintaining the competitiveness of the livestock farmers that satisfy the consumer’s demand for meat, milk and eggs. This supply is allowed by EC approval and supported by positive case by case EFSA Scientific Opinions and up to 16 years of environmental compatibility. Since EFSA has issued a positive Opinion on the cultivation of GM crops in the EU, what reasons can be provided to discriminate against European farmers who are not allowed to cultivate the same GM crops that are imported and consumed from other continents?
As discussed thoroughly also in the answer to question 6, this question is misleading when it states that livestock farmers need GM-soybean They need soybean, but it need not necessarily be transgenic. (a) As shown by USDA data on productivity of soybean in the U.S.A soybean production per acre steadily increased from 1977 to 2007 and the speed of increase did not change with the introduction of GM plants in 1996. (b) From the nutritional point of view, as far as we know (see answer to question 2) no data are available showing better results in animal feeding in the case of GM compared with non GM soybean. The reasons most of world wide soybean production stems from GM plants is the economic advantage coming from a reduction in the needed manpower for herbicide spraying on herbicide resistant cultivars and the control of the market by the three large holdings: Monsanto, Dupont and Syngenta. In our case the average size of farms is of 5–6 hectares; the farms with the extant GMPs may be up to more than hundred thousand. So, while in large farms airplanes can be utilized to spray herbicides, certainly our farmers have to rely on manpower working directly in the field. So here and in most anti-GMO European regions there is no manpower advantage.
There is no rational explanation for the EU’s current de facto ban on the cultivation of GM crops while concurrently allowing the import of GM produce from the Americas to prop up the meat, poultry and dairy industries.
The EU is a net importer of agricultural raw materials and 55 % of these imports come from ten countries, most of which have GM-based agricultural industries (Sabalza et al. 2011 ). Brazil, the United States and Argentina occupy the top three positions and are also the world’s largest GM producers, and almost all of the products imported from these countries are GM. The EC has recently proposed to give Member States the freedom to veto the cultivation of GM crops on their own territory without needing to provide any scientific evidence relating to new risks (European Commission 2010b ), ostensibly to prevent tactical voting leading to EU-wide bans (Casassus 2011 ). However, although the proposed amendment will allow member states to adopt measures against the cultivation of GM crops, they will not be allowed to prohibit the import or marketing of authorized GM products from elsewhere , which means that EU markets are likely to be flooded with imported GM products that could just as easily be home-grown. This is clearly a ludicrous position, which simultaneously restricts the freedom of EU farmers to grow the crops they choose and forces them to accept GM animal feed from abroad (Sabalza et al. 2011 ).
Even so, the import of GM products is also over-regulated, and this is particularly apparent in the EU’s treatment of imported corn and soybean from the United States, which has radically different regulations concerning adventitious presence limits, traceability and labeling (Ramessar et al. 2008a ). Although the EU is deficient in feed protein and is ultimately dependent on soybean imports, the complex and onerous process for approving imported GM products has discouraged overseas traders, resulting in a decline in imports from $2.8 billion in 1997 to $1.9 billion in 2008 (USDA 2009 ). This is despite EFSA issuing multiple Scientific Opinions declaring that GM products are safe and (as discussed above) the complete absence of any adverse effects of GM crops anywhere in the world throughout the 15+ years of cultivation.
A critical point is that if the EU continues to obstruct GM agriculture, it will force farmers to use environmentally hazardous, expensive and unsustainable agricultural practices, spend unnecessary resources on fossil fuels and agrochemicals, while at the same time importing GM products from the Americas. This policy will also discourage research and drive researchers overseas where the value chain can be realized in terms of released GM crops. Within the EU, researchers working on GM plants know that the best they can expect for their products is greenhouse cultivation, and that despite their benefits, GM crops are unlikely to be deployed in any setting where they could perform a useful function. Here the EU policy on GM crops is attacking its own foundations as a competitive bioeconomy because with one hand the EC offers funding for innovative biotech research and values (or even requires) the participation of small- to medium-sized enterprises (SMEs) and large industry partners, while with the other they prevent the same companies from realizing the value of their development pipeline. Many individual scientists and large companies with ambitious GM research projects have moved abroad to continue their work, and promising European SMEs have been unable to find investment partners (The Guardian 2003 , 2004 ). No significant investment in Europe is likely unless companies can recoup their R&D costs by selling their products to farmers. The attitude of European policymakers reveals the immense divide between the rational evaluation of science and business, and the panicky, expedient politics pandering to a populist media and activists (Farre et al. 2010 , 2011a , b ).
It is known that uncontrolled attacks of corn borers ( Ostrinia , Sesamia ) facilitate the growth of Fusarium moulds in corn grains leading to the accumulation of dangerous levels of fumonisins. The use of Bt corn has been proven to decrease/eliminate fumonisins from corn, and this is a contaminant that has led to European safety alerts and corn product recalls. What is your recommendation to reduce/eliminate mycotoxins in corn grain?
Of course there are more conventional methods to stop the attacks both through the use of chemicals and of biological agents, but certainly insect resistance may be a valid one when and if the plant is resistant to all corn borers at the same time and not only one of them, and of course the borers are not naturally selected for resistance to Bt toxins. Everybody who has been working in plant breeding knows that both in the case of “traditional” breeding and genetic engineering, insect resistant crops are resistant only for a short time because insects acquire resistance to the toxins in the case of genetic engineering or other genes leading to resistance in traditionally bred cultivars. This is happening in the case of maize in the USA and induced the Government to rule the maintenance of areas with susceptible plants to partially overcome this problem. In the case of cotton in China, the resistance to the boll worm induced the multiplication of more than a hundred competitor species, and therefore the amount of insecticides rose to levels never reached with non-boll worm resistant crops
Fumonisins are mycotoxins produced by Fusarium molds when they colonize cereal grains. They are toxic to humans, particularly affecting liver and kidney functions, causing esophageal cancer, increasing HIV transmission rates (Williams et al. 2010 ) and inducing neural tube defects such as spina bifida in utero (Marasas et al. 2004 ; Torres et al. 2007 ). The maximum tolerable daily intake is 2 μg/kg body weight as stated in EC Regulation 1881/2006. Many nations have established regulatory standards stating maximum tolerance levels for mycotoxins in food and feed. Therefore, aside from the health risks described above, mycotoxin contamination can also reduce the price paid for food crops, or in extreme cases, can cause market rejection of entire food or feed shipments (Wu et al. 2004 ; Wu 2006 ). The maximum permitted daily intake of fumonisins was doubled in EC Regulation 1126/2007 in recognition of the fact that recommended levels cannot be achieved under some circumstances. This is not a recommended practice, nor is it consistent with other EC decisions including the application of the precautionary approach, because several corn herbicides have been banned in the EU at contamination levels far lower than allowed for fumonisins (Wu 2006 ).
There is a clear relationship between corn borer damage and unsafe levels of fumonisins in raw corn, reflecting the penetration of damaged corn kernels by the fungus (Munkvold et al. 1997 ; Ariño 2009 ; Escobar and Quintana 2008 ; EFSA 2005 ). Any method that reduces insect damage in corn also reduces the risk of fungal contamination, but foliar Bt sprays are not sufficient because the corn borers are protected inside the cob (Sanahuja et al. 2011 ). Bt corn confers resistance to corn borers and therefore reduces mycotoxin contamination. In Europe and elsewhere, field trials of Bt corn on 288 separate test sites have shown that harvested kernels have significantly lower fumonisin levels than non-Bt counterparts, with fumonisin concentrations in Bt grain usually lower than 4 μg/kg and often below 2 μg/kg (Wu 2006 ). Interestingly, 31 % of fumonisin contamination alerts in Spanish corn grain represent organically-grown corn, which represents less than 1 % of the area under cultivation, and the other 69 % represent conventional corn. No alerts have been raised for borer-resistant GM corn, which represents 21 % of the cultivated area. This information comes directly from the Spanish Ministry of the Environment ( http://www.efsa.europa.eu/en/events/documents/gmo090914-p13.pdf ).
Similar indications come from import checks in Italy where contamination in Bt corn is consistently registered as lower than conventional corn. The benefit of Bt corn in terms of the reduction of mycotoxin damage has been virtually ignored in policy debates, despite its positive economic impact in the US and its effect on both health and the economy in developing countries (Wu et al. 2004 ; Wu 2006 ). In my opinion its cultivation should be mandatory in EU regions where corn borers are endemic, but the cultivation of Bt corn is subject to a de facto ban across large areas of the EU and particularly for nations such as Italy (Table 1 ) and France where fumonisin toxicity is prevalent (Pietri and Piva 2000 ; Masoero et al. 1999 ; Folcher et al. 2010 ).
Roughly one quarter of the Earth’s terrestrial surface is now under cultivation with more land converted to crop production in the 30 years after 1950 than in the previous 150 years. Given this picture, economic and social concerns present critical challenges to agriculture in the next decades. Farm profitability, viability of rural communities, fair trade and agricultural labor represent significant issues. Which are the advantages of GM crops for agriculture from the economic and social points of view?
GM crops provide tools that are compatible with many of the other approaches used currently to increase food production, while reducing the environmental footprint of agriculture and increasing the affordability of crops (Christou and Twyman 2004 ). The socioeconomic advantages of GM crops are demonstrated by the consistent growth in adoption since the first commercial releases (James 2010 ) combined with ample evidence of greater farm profitability in both developed economies like the US (Smale et al. 2009 ) and emerging economies like India (Subramanian and Qaim 2010 ). There has been considerable debate about economic potential of GM crops in developing countries (Park et al. 2011 ), and an extensive analysis carried out by Brookes and Barfoot ( 2010 ) showed that approximately two thirds of the net benefits of GM agriculture go to farmers, and one third to the seed supply chain. In the case of Bt crops, these benefits include yield improvements, higher revenues and lower pesticide costs, which more than compensate for the higher seed prices.
Overall, the available evidence confirms that in both developed and developing countries, the adoption of GM crops can increase the farmer’s income. The increase in income to small-scale farmers in developing countries can have a direct impact on poverty alleviation and quality of life, a key component of sustainable development. Bennett et al. ( 2006 ) compared the performance of Bt and non- Bt cotton in resource-poor smallholder cotton farm plots in India and South Africa. Their results demonstrated that in many agricultural environments the adopters of Bt cotton benefit in terms of higher yields, reduced labor and pesticide use, and ultimately higher gross margins per hectare, leading them to conclude that ‘that the smallest producers are shown to have benefited from adoption of the Bt variety as much as, if not more than, larger producers.’
Even where economic issues of coexistence come into play, smallholder farmers usually trade their GM and non-GM crops together, using cooperatives or local dealers that also provide seeds and other inputs. In this way, in corn-borer endemic areas where Bt-corn is approved and its use makes sense, it is common to see 50–80 % of farmers using GM corn, without isolation barriers and only the required refuges of non-GM corn to delay the appearance of resistant corn borer strains. These jointly marketed products are labelled as GM corn even if only 50–80 % of the grain is transgenic.
The social impact of GM agriculture is intertwined with the economic benefits because the higher margins generated by GM crops help efforts to alleviate poverty, and therefore provide better access to food, medicine and education, enhancing the social dimension of sustainability (Yuan et al. 2011 ). Second- and third-generation GM crops have been developed to address these issues directly by improving nutrition or providing inexpensive drugs, but even the first-generation crops have indirectly led to improvements simply by increasing the profitability of farms and empowering the smallholders in a socioeconomic context. There have also been more direct health benefits of GM agriculture by reducing exposure to pesticides (Brimner et al. 2005 ; Knox et al. 2006 ), changing the patterns of herbicide use to favor those with lower toxicity such as glyphosate, and as mentioned above, reducing the exposure of populations to mycotoxins (Munkvold et al. 1999 ). Work is also well advanced in the development of GM crops that will have a direct impact on health, e.g. those with reduced allergens (Chu et al. 2008 ), higher levels of proteins and carbohydrates (reviewed by Newell-McGloughlin 2008 ), and higher levels of essential amino acids, essential fatty acids, vitamins and minerals (Damude and Kinney 2008 ), the most prevalent examples being Golden Rice (Potrykus 2010 ), multivitamin corn (Naqvi et al. 2009 ; Zhu et al. 2008 ), and high zeaxanthin corn (Naqvi et al. 2011b ). GM agriculture can therefore have a significant impact on both industrialized and developing economies by increasing farm profit margins, as well as by contributing to the social dimension of sustainable development by reducing the handling and use of pesticides, exposure to adventitious mycotoxins and, ultimately, by directly addressing the causes of hunger and malnutrition.
As I mentioned before, the productivity of maize and soybean, according to USDA data from 1977 to 2007, did not increase from the introduction of GM-crops but probably from the improvements in management and conventional breeding. Moreover, in the case of Bt, the advertised reduction in the usage of insecticides did not happen because of the selection of Bt resistant insects and the fast reproduction of other parasite species than those killed by Cry toxins, as we shall discuss later. Also the cost of herbicides has not been obviously reduced, the very aim of herbicide resistant plant introduction being an increased number of treatments also during plant growth. Therefore, as already mentioned, the economic advantage of the introduction of herbicide resistance traits is the reduction of manpower costs, all this favouring farms of large dimensions with an increase of the input of capital and a decrease of labour leading to the exit from the systems of subsistence agriculture due to lack of capital. The reasons of the outstanding success of GM crops particularly in the USA, Canada and Latin-American countries can be understood only if we look at the structure of the market for the four mentioned crops. In the first place, (for a good review, see Howard 2009 ) since the nineteen-nineties, a very fast concentration process has occurred, few multinational companies gaining the control of large part of the food related market, the first four companies controlling 59 % of the pesticides, 56 % of the seed and practically all GMPs. This process has been favoured by the extension, within the TRIPS agreement, of industrial patents to living objects and processes and by the change in the UPOV cancelling both the so-called farmer’s and breeder’s rights. To give an idea of the power given to the holders of patents, already in 1995, according to the World Patent Index, Bt maize was covered by 440 patents, 88 % of which were owned by industry. Nowadays, three companies, through IPRs, have the control not only of GMPs but also of innovations related to other steps of the food production chain. That follows from the fact that all the leading companies, before GMO production, were agro-chemical industries and since the 1990s acquired control of chemical, pharmaceutical and seed companies. For instance, Monsanto, a herbicide producer in the sixties, acquired Pharmacia and Upjohn and the seed industries Cargill, Dekalb Genetics Corporation, Delta and Pine Land, Seminis, and Holden Foundation Seeds and controls more than 200 seed companies in India, China and Brazil. Dupont, on the other hand, has acquired the seed company Pioneer High Bred, while Syngenta derived from the fusion between Novartis agriculture and Zeneca. The power of the leading companies is also based on the presence in public control agencies and in the editorial boards of international scientific journals, as thoroughly discussed by Glover ( 2009 ), on behalf of the British E.S.R.C., in his critical synthesis of the scientific literature concerning Bt-Cotton in China, India, South Africa. For this reason, according to Glover, it is not widely known that in China BT cotton is useful only in the case of heavy presence of the boll-worm, that insecticide consumption does not decrease (Wang 2008 ). Nor it is known that in India, in the regions of Andhra Pradesh and Maharashtra, the presence of 150 different species of insects obliged the farmers to increase the input of pesticides while the price of cotton was decreasing (Ramasundaram et al. 2007 ). In Latin America, on the other hand, problems derived from the transformation of local subsistence agricultures based on the production of food into industrial farming, aimed at the export of soybean for animal feeding in developed countries. In Argentina, Brazil, Paraguay and, lately also Uruguay, many small farms were purchased and replaced by large ones, up to 100.000 hectares. For this reason, in Argentina, soybean production rose from 1996 to 2004 by 11.8 %, that of wheat being -2.3 % lower, potato −3.3 %, millet −19.1 % and labour also being reduced by 50 % (Gallacher 2009 ). In Brazil, farmers were expelled with the use of force and the big soy producers from Argentina along with Japanese and German jobbers control 76 % of Paraguay soybean producers, thus further reducing revenues and jobs. Of course, in all these cases the economic and social disasters deriving from the introduction of GMPs were not due by any means to genetic engineering techniques as such, but by the structure of the market where for the first time living objects could be covered by industrial patents through economical and political agreements between the producer companies and governments and under the rules of the WTO. However, it is worth stressing here that, as mentioned before, the advantages of GMPs only favoured large farms and the multinational companies, small farmers leaving the fields and the seeds of a number of relevant crops and losing languages and traditional knowledge in the favelas of several countries (see the data in the website of Terralingua, an NGO working on bio-linguistic problems).
The possible economic advantages of GMOs in an agricultural context have been discussed extensively. In this respect GMOs have been viewed by some as an effective way to meet the energy needs of the most vulnerable, malnourished populations in developing countries. Do the available results provide indications for a possible role of GMOs in improving food quality, therefore providing specific nutritional advantages also in wealthy population groups?
The improvement of nutritional quality of crops has been one of the main objectives of plant genetic engineering as, in theory, the modification of metabolic pathways could lead to the qualitative and quantitative improvement of specific nutritional components. Rather unfortunately, due to the network structure of plant metabolism implying that a change in one node will affect other components, the results have been far from successful. As far as I remember, the only putative success has been obtained with the so-called “Golden Rice”, a producer of pro-vitamin A which was released into the market but soon withdrawn because of the low level of production of the molecule. This happened a few years ago and we are now waiting for new cultivars with improved production. Unfortunately, as already discussed, the research intensity of GMO producers has been lower and lower, thus slowing the release of really innovative cultivars in all fields.
GMOs certainly have the potential to provide nutritional advantages for wealthy population groups, despite the controversy about GM agriculture in Europe discussed elsewhere in this article. One of the important benefits of transgenic crops is the ability to generate more nutritious varieties, and although these are currently targeted towards developing countries with the worst malnutrition levels, they offer clear benefits to all sectors of the population. Even in Europe there is a surprisingly large malnourished population, which has arisen not only through the impact of poverty but also through ignorance and poor lifestyle choices. Malnourishment is particularly rife in the elderly population because one of the consequences of aging is a progressive loss of the ability to absorb nutrients (Ljungqvist et al. 2010 ).
EU policies on food and nutrition are described in the European Commission White Paper on Food Safety and the Program for Public Health (European Commission 2000 ). The fortification of processed food and agronomic biofortification using nutrient-rich fertilizers have been applied successfully to overcome the lower levels of nutrients in the UK and Finland (Lyons et al. 2003 ; Broadley et al. 2006 ), but there are also several sectors of the wealthy population where nutritional and food quality needs could be met through the use of transgenic crops. The most significant is the biofortification of cereals, legumes, fruits and vegetables with iron to combat anemia resulting from iron deficiency caused by poor dietary habits (Lucca et al. 2002 ). This is because traditional routes such as iron supplements can be inefficient because of poor compliance (Darnton-Hill and Nalubola 2002 ; Gómez-Galera et al. 2010 ). Another interesting example is the potential to increase the carotenoid levels in cereals such as maize to address macular degeneration in the elderly. Most people know that β-carotene is required in the diet as a source of vitamin A, but few recognize the importance of other carotenoids such as lutein and zeaxanthin, which are required in the eye to prevent damage caused by strong light (Landrum and Bone 2001 ). A diet rich in these molecules has been linked with eye health in the ageing population and biofortification at source would be an advantageous way to address this growing problem (Hammond et al. 1997 ; Landrum et al. 1997 ).
Another valuable approach is the fortification of staple foods such as cereals with polyunsaturated fatty acids currently only found in fish. The metabolic pathways that lead to omega fatty acids are understood and can be recreated in plants (Ye and Bhatia 2012 ). The development of cereal products enriched with these essential fatty acids would increase the general health of the population by providing essential nutrients to those who rarely eat fish, and would also reduce pressure on fish stocks as a sole source of this nutrient.
The controversy surrounding mycotoxin levels in maize is discussed in another section, but it is worth pointing out here that this is a problem that faces all consumers, not just those in developing countries, so the ability to grow Bt maize commercially in Europe would, again, provide consumers from all population groups with higher quality food and would at the same time remove the need to import exactly the same products from abroad (Folcher et al. 2010 ).
Finally, there is a great deal of interest in the development of functional foods that provide added-value health benefits to consumers as well as calories (e.g. antioxidants and other health-promoting compounds). Since the metabolic pathways leading to many of these valuable molecules are now being unraveled, it is likely that the first generation of biofortified foods containing essential nutrients will be followed by a second wave of luxury goods aimed at the higher-income sectors, comprising food products with enhanced levels of health-promoting compounds (Zhu et al. 2012 ).
Conclusions
There is obviously no final conclusion of this debate, which is likely to continue for years. We can foresee that plant biotechnology will potentially be able to provide several benefits and address many challenges in food production. However, it is also crucial that the release of GM crops in the environment does not bear new risks and irretrievable consequences and/or threats for human health.
However, within this framework, it would be desirable to reach a global harmonization of regulation and legislation of GM crops in order to face the ongoing globalisation of agricultural production. GM crops, in fact, are going to become significant in world crop production as the cultivation of GM crops in the world in 2011 reached 160 million hectares (+8 % with respect to 2010, a 94-fold increase with respect to 1996) in 29 countries worldwide. According to the International Service for the Acquisition of Agri-biotech Applications (James 2011 ), 16.7 million farmers grew biotech crops in 2011, over 90 % were small resource farmers in developing countries (7 million in China and 7 million in India), and they collectively planted 14.5 million hectares of GM crops. The US is the lead producer of GM crops, with 69.0 million hectares (maize, soybean, cotton, canola, sugarbeet, alfalfa, papaya, squash) followed by Brazil (30.3 million hectares, soybean, maize, cotton), Argentina (23.7 million hectares, soybean, maize, cotton), India (10.6 million hectares, cotton), Canada (10.4 million hectares, canola, maize, soybean, sugarbeet) and China (3.9 million hectares, cotton, papaya, poplar, tomato, sweet pepper). In Europe, six EU countries (Spain, Portugal, Czechia, Poland, Slovakia and Romania) planted 114.490 hectares of Bt maize (+26 % as respect to 2010), with Spain growing 85 % of the total in the EU.
Obviously, this paper is not aimed at reaching any conclusion on this controversial matter. However, we hope that this face-to-face between two almost opposite positions can contribute to the discussion related to this delicate aspect of agro-food science.
It is worth stating that the debates on international markets, economical issues, crop productivity, ethical aspects and environmental concerns are indisputably important, but only keeping in mind that the first, most imperative issue is to warrant a reliable, safe and healthy nutrition to the population.
Ariño A (2009) Influence of agricultural practices on the contamination of corn by fumonisin mycotoxins. J Food Protect 72:898–902
Google Scholar
Bai C, Twyman RM, Farre G, Sanahuja G, Christou P, Capell T, Zhu C (2011) A golden era—pro-vitamin A enhancement in diverse crops. In Vitro Cell Dev Biol Plants 47:205–221
Article CAS Google Scholar
Barriuso J, Marín S and Mellado RP (2010) Effect of the herbicide glyphosate on glyphosate-tolerant corn rhizobacterial communities: a comparison with pre-emergency applied herbicide consisting of a combination of acetochlor and terbuthylazine. Environ Microbiol 12(4):1021–1030
Barriuso J, Valverde JR, Mellad RP (2011a) Effect of the herbicide glyphosate on the culturable fraction of glyphosate-tolerant corn rhizobacterial communities using two different growth media. Microbes Environ 26:332–338
Article PubMed Google Scholar
Barriuso J, Marín S, Mellado RP (2011b) Potential accumulative effect of the herbicide glyphosate on glyphosate-tolerant corn rhizobacterial communities over a three-year cultivation period. PLoS One 6(11):e27558. doi: 10.1371/journal.pone.0027558
Article PubMed CAS Google Scholar
Batista R, Oliveira MM (2009) Facts and fiction of genetically engineered food. Trends Biotechnol 27:277–286
Beckie HJ (2009) Field trials prove speed of herbicide resistance. Western Producer, 1 Oct 2009, p 65
Belmonte J (1993) Estudio comparativo sobre la influencia del laboreo en las poblaciones de vertebrados en la campiña de Jerez. Bol San Veg Plagas 19:211–220
Bennett R, Morse S, Ismael Y (2006) The economic impact of genetically modified cotton on South African smallholders: yield, profit and health effects. J Dev Stud 42:662–677
Article Google Scholar
Brimner TA, Gallivan GJ, Stephenson GR (2005) Influence of herbicide-resistant canola on the environmental impact of weed management. Pest Manag Sci 61:47–52
Broadley MR, White PJ, Bryson RJ, Meacham MC, Bowen HC, Johnson SE, Hawkesford MJ, McGrath SP, Fang-Jie Z, Breward N, Harriman M, Tucker M (2006) Biofortification of UK food crops with selenium. Proc Nutr Soc 65:169–181
Brookes G, Barfoot P (2009) Global impact of biotech crops: income and production effects, 1996–2007. AgBioForum 12:184–200
Brookes G, Barfoot P (2010) GM crops: global socio-economic and environmental impacts 1996–2008. PG Economics Ltd, Dorchester
Casassus B (2011) EU parliament votes to allow restrictions on GM food. Nature News Blog, 6 July 2011 ( http://blogs.nature.com/news/2011/07/eu_parliament_votes_to_allow_r.html )
Castaldini M, Turrini A, Sbrana C, Benedetti A, Marchionni M, Mocali S, Fabiani A, Landi S, Santomassimo F, Pietrangeli B, Nuti MP, Miclaus N, Giovannetti M (2005) Impact of Bt corn on rhizospheric and soil eubacterial communities and on beneficial mycorrhizal symbiosis in experimental microcosms. Appl Environ Microbiol 71:6719–6729
Christou P, Twyman RM (2004) The potential of genetically enhanced plants to address food insecurity. Nutr Res Rev 17:23–42
Christou P, Capell T, Kohli A, Gatehouse JA, Gatehouse AMR (2006) Recent developments and future prospects in insect pest control in transgenic crops. Trends Plant Sci 11:302–308
Chu Y, Faustinelli P, Ramos ML, Hajduch M, Stevenson S, Thelen JJ, Maleki SJ, Cheng H, Ozias-Akins P (2008) Reduction of IgE binding and nonpromotion of Aspergillus flavus fungal growth by simultaneously silencing Ara h 2 and Ara h 6 in peanut. J Agric Food Chem 56:11225–11233
Collinge DB, Jørgensen HJ, Lund OS, Lyngkjaer MF (2010) Engineering pathogen resistance in crop plants: current trends and future prospects. Annu Rev Phytopathol 48:269–291
Cominelli E, Tonelli C (2010) Transgenic crops coping with water scarcity. Nat Biotechnol 27:473–477
CAS Google Scholar
Conner AJ, Glare TR, Nap JP (2003) The release of genetically modified crops into the environment part II. Plant J 33:19–46
Crawley MJ, Brown SL, Hails RS, Kohn DD, Rees M (2001) Biotechnology: transgenic crops in natural habitats. Nature 409:682–683
Damude HG, Kinney AJ (2008) Engineering oilseed plants for a sustainable, land-based source of long chain polyunsaturated fatty acids. Lipids 42:179–185
Darnton-Hill I, Nalubola R (2002) Fortification strategies to meet micronutrient needs: successes and failures. Proc Nutr Soc 61:231–241
EC Research (2001) EC-sponsored research on safety of genetically modified organisms: a review of results: http://europa.eu.int/comm/research/quality-of-life/gmo/
EFSA (2005) Opinion of the scientific panel on contaminants in food chain on a request from the commission related to fumonisins as undesirable substances in animal feed. EFSA J 235:1–32
EFSA (2010) Guidance on the environmental risk assessment of genetically modified plants. EFSA J 8:1879
Escobar J, Quintana J (2008) Reducción de riesgos sanitarios con el cultivo de un maíz transgénico. Libro de Resúmenes XIII Congreso Anual en Ciencia y Tecnología de los Alimentos, pp 29–31
European Commission (2000) White paper on food safety
European Commission (2010) A decade of EU-funded GMO research. Food, agriculture & fisheries & biotechnology, European Research Area, European Commission, Brussels, p 18
European Commission (2010) Memo 10/325: Questions and answers on the EU’s new approach to the cultivation of GMOs. European Commission, Brussels
Farre G, Ramessar K, Twyman RM, Capell T, Christou P (2010) The humanitarian impact of plant biotechnology: recent breakthroughs vs bottlenecks for adoption. Curr Opin Plant Biol 13:219–225
Farre G, Bai C, Twyman RM, Capell C, Christou P, Zhu C (2011a) Nutritious crops producing multiple carotenoids—a metabolic balancing act. Trends Plant Sci 16:532–540
Farre G, Twyman RM, Zhu C, Capell T, Christou P (2011b) Nutritionally enhanced crops and food security: scientific achievements versus political expediency. Curr Opin Biotechnol 22:245–251
Ferry N, Edwards MG, Gatehouse JA, Capell T, Christou P, Gatehouse AMR (2006) Transgenic plants for insect pest control. A forward looking scientific perspective. Transgen Res 15:13–19
Folcher L, Delos M, Marengue E, Jarry M, Weissenberger A, Eychenne N, Regnault-Roger C (2010) Lower mycotoxin levels in Bt corn grain. Agron Sustain Dev 30:711–719
Gallacher M (2009) The changing structure of production: argentine agriculture 1988–2002, Universidad del CEMA, Buenos Aires, Documento de trabajo 415
Giovannetti M, Sbrana C, Turrini A (2005) The impact of genetically modified crops on soil microbial communities. Biol Forum 98:393–418
Glover D (2009) Undying promise: agricultural biotechnology’s pro-poor narrative, ten years on. STEPS Centre ESRC, UK
Gómez-Galera S, Rojas E, Sudhakar D, Zhu C, Pelacho AM, Capell T, Christou P (2010) Critical evaluation of strategies for mineral fortification of staple food crops. Transgenic Res 19:165–180
Gressel J (2002) Molecular biology of weed control. Taylor and Francis, Abington, p 520
Gressel J (2008) Genetic glass ceilings—transgenics for crop biodiversity. Johns Hopkins University Press, Baltimore, p 461
Gressel J (2012) Containing and mitigating transgene flow from crops to weeds, to wild species, and to crops. In: Altman A, Hasegawa PM (eds) Plant biotechnology and agriculture: prospects for the 21st century. Elsevier Press, NY, pp 509–523
Chapter Google Scholar
Hammond BR Jr, Johnson EJ, Russell RM, Krinsky NI, Yeum KJ, Edwards RB, Snodderly DM (1997) Dietary modification of human macular pigment density. Invest Ophthalmol Vis Sci 38:1795–1801
PubMed Google Scholar
Hare PD, Chua NH (2002) Excision of selectable marker genes from transgenic plants. Nat Biotechnol 20:575–580
Howard PH (2009) Visualizing consolidation in the global seed industry. Sustainability 1(4):1266–1287
Huber DM (2010) Agro-chemical and crop nutrient interactions: current update. Proc Fluid Fert Forum Scottsdale 27:1–13
Hutchison WD, Burkness EC, Mitchell PD, Moon RD, Leslie TW, Fleischer SJ, Abrahamson M, Hamilton KL, Steffey KL, Gray ME, Hellmich RL, Kaster LV, Hunt TE, Wright RJ, Pecinovsky K, Rabaey TL, Flood BR, Raun ES (2010) Areawide suppression of European corn borer with Bt corn reaps savings to non-Bt corn growers. Science 330:222–225
Huvenne H, Smagghe G (2010) Mechanisms of dsRNA uptake in insects and potential of RNAi for pest control: a review. J Insect Physiol 56:227–235
Icoz I, Stotzky G (2008) Fate and effects of insect-resistant Bt crops in soil ecosystems. Soil Biol Biochem 40:559–586
James C (2010) Global status of commercialized biotech/GM crops: 2009. ISAAA Brief 41-2009. ISAAA, Ithaca
James C (2011) Global status of commercialized biotech/GM crops: 2011. ISAAA Brief 43-2011. ISAAA, Ithaca
Kessler C, Economidis I (2001) EC-sponsored research on safety of genetically modified organisms. European Commission. Community Research
Knox OGG, Vadakattu GVSR, Gordon K, Lardner R, Hicks M (2006) Environmental impact of conventional and Bt insecticidal cotton expressing one and two Cry genes in Australia. Aust J Agr Res 57:501–509
Kremer RG, Means NE (2009) Glyphosate and glyphosate-resistant crop interactions with rhizosphere microorganisms. Eur J Agron 31:153–161
Landrum JT, Bone RA (2001) Lutein, zeaxanthin, and the macular pigment. Arch Biochem Biophys 385:28–40
Landrum JT, Bone RA, Joa H, Kilburn MD, Moore LL, Sprague KE (1997) A one year study of the macular pigment: the effect of 140 days of a lutein supplement. Exp Eye Res 65:57–62
Ljungqvist O, Gossum AV, Sanz ML, Man F (2010) The European fight against malnutrition. Clin Nutr 29:149–150
Lucca P, Hurrell R, Potrykus I (2002) Fighting iron deficiency anemia with iron-rich rice. J Am Coll Nutr 21:184S–190S
PubMed CAS Google Scholar
Lyons G, Stangoulis J, Graham R (2003) High-selenium wheat: biofortification for better health. Nutr Res Rev 16:45–60
Ma JKC, Drake PMW, Christou P (2003) The production of recombinant pharmaceutical proteins in plants. Nat Rev Genet 4:794–805
Ma JKC, Barros E, Bock R, Christou P, Dale PJ, Dix PJ, Fischer R, Irwin J, Mahoney R, Pezzotti M, Schillberg S, Sparrow P, Stoger E, Twyman RM (2005) Molecular farming for new drugs and vaccines. Current perspectives on the production of pharmaceuticals in transgenic plants. EMBO Rep 6:593–599
Marasas et al (2004) Fumonisins disrupt sphingolipid metabolism, folate transport, and neural tube development in embryo culture and invivo: a potential risk factor for human neural tube defects among populations consuming fumonisin-contaminated corn. J Nutr 134:711–716
Masoero F, Moschini M, Rossi F, Prandini A, Pietri A (1999) Nutritive value, Mycotoxin contamination and in vitro rumen fermentation of normal and genetically modified corn (cry 1A(b)) grown in northern Italy. Maydica 44:205–209
Mehlo L, Gahakwa D, Nghia PT, Loc NT, Capell T, Gatehouse JA, Gatehouse AMR, Christou P (2005) An alternative strategy for sustainable pest resistance in genetically enhanced crops. Proc Natl Acad Sci USA 102:7812–7816
Morris HS, Spillane C (2010) EU GM crop regulation: a road to resolution or a regulatory roundabout? Symposium on the EU’s GMO reform. Eur J Risk Reg 4:359–369
Munkvold GP, Hellmich RL, Showers WB (1997) Reduced Fusarium ear rot and symptomless infection in kernels of corn genetically engineered for European corn borer resistance. Phytopathology 87:1071–1077
Munkvold GP, Hellmich RL, Rice LG (1999) Comparison of fumonisin concentrations in kernels of transgenic Bt corn hybrids and non-transgenic hybrids. Plant Dis 81:556–565
Nap JP, Metz PLJ, Escaler M, Conner AJ (2003) The release of genetically modified crops into the environment part I. Plant J 33:1–18
Naqvi S, Zhu C, Farre G, Ramessar K, Bassie L, Breitenbach J, Perez Conesa D, Ros G, Sandmann G, Capell T, Christou P (2009) Transgenic multivitamin corn through biofortification of endosperm with three vitamins representing three distinct metabolic pathways. Proc Natl Acad Sci USA 106:7762–7767
Naqvi S, Ramessar K, Farre G, Sabalza M, Miralpeix B, Twyman RM, Capell T, Christou P, Zhu C (2011a) High value products from transgenic corn. Biotechnol Adv 29:40–53
Naqvi S, Zhu C, Farre G, Sandmann G, Capell T, Christou P (2011b) Synergistic metabolism in hybrid corn indicates bottlenecks in the carotenoid pathway and leads to the accumulation of extraordinary levels of the nutritionally important carotenoid zeaxanthin. Plant Biotechnol J 9:384–393
Newell-McGloughlin M (2008) Nutritionally improved agricultural crops. Plant Physiol 147:939–953
Park RJ, McFarlane I, Phipps RH, Ceddia G (2011) The role of transgenic crops in sustainable development. Plant Biotechnol J 9:2–21
Peremarti A, Twyman RM, Gomez-Galera S, Naqvi S, Farre G, Sabalza M, Miralpeix B, Dashevskaya S, Yuan D, Ramessar K, Christou P, Zhu C, Bassie L, Capell T (2010) Promoter diversity in multigene transformation. Plant Mol Biol 73:363–378
Pietri A, Piva G (2000) Occurrence and control of mycotoxins in corn grown in Italy. Proceedings of the VI international feed production conference, pp 226–236, Piacenza, Nov 27–28
Potrykus I (2010) Lessons from the ‘Humanitarian Golden Rice’ project: regulation prevents development of public good genetically engineered crop products. Nat Biotechnol 27:466–472
Prischl M, Hackl E, Pastar M, Pfeiffer S, Sessitsch A (2012) Genetically modified Bt corn lines containing cry3Bb1, cry1A105 or cry1Ab2 do not affect the structure and functioning of root-associated endophyte communities 54:39–48
Ramasundaram P, Vennila S, Ingle RK (2007) Bt cotton performance and constraints in Central India. Outlook Agric 36(3):175–180
Ramessar K, Peremarti A, Gomez Galera S, Naqvi S, Moralejo M, Muñoz M, Capell T, Christou P (2007) Biosafety and risk assessment framework for selectable marker genes in transgenic crop plants. A case of the science not supporting the politics. Transgen Res 16:261–280
Ramessar K, Capell T, Twyman RM, Quemada H, Christou P (2008a) Trace and traceability—a call for regulatory harmony. Nat Biotechnol 26:975–978
Ramessar K, Rademacher T, Sack M, Stadlmann J, Platis D, Stiegler G, Labrou N, Altmann F, Ma J, Stöger E, Capell T, Christou P (2008b) Cost-effective production of a vaginal protein microbicide to prevent HIV transmission. Proc Natl Acad Sci USA 105:3727–3732
Ramessar K, Sabalza M, Capell T, Christou P (2008c) Corn plants: an ideal production platform for effective and safe molecular pharming. Plant Sci 174:409–419
Ramessar K, Capell T, Twyman RM, Quemada H, Christou P (2009) Calling the tunes on transgenic crops—the case for regulatory harmony. Mol Breed 23:99–112
Ramessar K, Capell T, Twyman RM, Christou P (2010) Going to ridiculous lengths—European coexistence regulations for GM crops. Nat Biotechnol 28:133–136
RF Service (2007) A growing threat down on the farm. Science 316:1114–1117
Ricroch A, Bergé JB, Kuntz M (2010) Is the German suspension of MON810 corn cultivation scientifically justified? Transgenic Res 19:1–12
Romeis J, Meissle M, Bigler F (2006) Transgenic crops expressing Bacillus thuringiensis toxins and biological control. Nat Biotechnol 24:63–71
Rosati A, Bogani P, Santarlasci A, Buiatti M (2008) Characterisation of 3’transgene insertion site and derived mRNAs in MON810 yield gard maize. Plant Mol Biol 67:271–281
Sabalza M, Miralpeix B, Twyman RM, Capell T, Christou P (2011) EU legitimizes GM crop exclusion zones. Nat Biotechnol 29:315–317
Sanahuja G, Subhasappa RB, Twyman RM, Capell T, Christou P (2011) Bacillus thuringiensis—a century of research, development and commercial applications. Plant Biotechnol J 9:283–300
Schimmelpfennig DE, Pray CE, Brennan MF (2004) The impact of seed industry concentration on innovation: a study of US biotech market leaders. Agric Econ 30:157–167
Sears MK, Hellmich RL, Stanley-Horn DE, Oberhauser KS, Pleasants JM, Mattila HR, Siegfried BD, Dively GP (2001) Impact of Bt corn pollen on monarch butterfly populations: a risk assessment. Proc Natl Acad Sci USA 98:11937–11942
Shelton AM, Sears MK (2001) The monarch butterfly controversy: scientific interpretations of a phenomenon. Plant J 27:483–488
Smale M, Zambrano P, Gruère G, Falck-Zepeda J, Matuschke I, Horna D, Nagarajan L, Yerramareddy I, Jones H (2009) Measuring the economic impacts of transgenic crops in developing agriculture during the first decade. IFPRI Food Policy Reviews, IFPRI, Washington DC
Snow A (2009) Unwanted transgenes re-discovered in oaxacan maize. Mol Ecol 18:569–571
Stoger E, Ma JK, Fischer R, Christou P (2005) Sowing the seeds of success: pharmaceutical proteins from plants. Curr Opin Biotechnol 16:167–173
Subramanian A, Qaim M (2010) The impact of Bt cotton on poor households in rural India. J Dev Stud 46:295–311
Sussman GL, Tarlo S, Dolovich J (1991) The spectrum of IgE-mediated responses to latex. JAMA 265:2844–2847
Svitashev SK, Somers DA (2001) Genomic interspersions determine the size and complexity of transgene loci in transgenic plants produced by microprojectile bombardment. Genome 44:691–697
Tebrügge F (2010) No-tillage visions—protection of soil, water and climate and influence on management and farm income. In: García-Torres L, Benites J, Martínez-Vilela A, Holgado-Cabrera A (eds) Conservation agriculture: environment, farmers experiences, innovations, socio-economy, policy. Springer, NY, pp 327–340
The Guardian (2003) Brain drain threatens GM crop research. The Guardian, 23-09-2003
The Guardian (2004) Syngenta moves GM research to America. The Guardian, 02-07-2004
Torres et al (2007) Estimated fumonisin exposure in Guatemala is greatest in consumers of lowland corn. J Nutr 137:2723–2729
Twyman RM, Ramessar K, Quemada H, Capell T, Christou P (2009) Plant biotechnology: the importance of being accurate. Trends Biotechnol 27:609–612
USDA (2009) US Department of Agriculture GAIN Report: EU-27 Biotechnology. GE Plants and Animals. USDA, Washington, DC
Wang S (2008) Bt cotton and secondary pests. Int J Biotechnol 10(2–3):113–121
Weinthal D, Tovkach A, Zeevi V, Tzfira T (2010) Genome editing in plant cells by zinc finger nucleases. Trends Plant Sci 15:308–321
Williams et al (2010) HIV and hepatocellular and esophageal carcinomas related to consumption of mycotoxin-prone foods in sub-Saharan Africa. Am J Clin Nutr 92:154–160
Wu F (2006) Mycotoxin reduction in Bt corn: potential economic, health, and regulatory impacts. Transgen Res 15:277–289
Wu F, Miller JD, Casman EA (2004) The economic impact of Bt corn resulting from mycotoxin reduction. J Toxicol 23:397–424
Ye VM, Bhatia SK (2012) Metabolic engineering for the production of clinically important molecules: omega-3 fatty acids, artemisinin, and taxol. Biotechnol J 7:20–33
Yuan D, Bassie L, Sabalza M, Miralpeix B, Dashevskaya S, Farre G, Rivera SM, Subhasappa RB, Bai C, Sanahuja G, Arjo G, Avilla E, Zorrilla-Lopez U, Ugido N, Lopez A, Almacellas D, Zhu C, Capell T, Hahne G, Twyman RM, Christou P (2011) The potential impact of plant biotechnology on the millennium development goals. Plant Cell Rep 30:249–265
Zhu C, Naqvi S, Gomez-Galera S, Pelacho AM, Capell T, Christou P (2007) Transgenic strategies for the nutritional enhancement of plants. Trends Plant Sci 12:548–555
Zhu C, Naqvi S, Breitenbach J, Sandmann G, Christou P, Capell T (2008) Combinatorial genetic transformation generates a library of metabolic phenotypes for the carotenoid pathway in corn. Proc Natl Acad Sci USA 105:18232–18237
Zhu C, Sanahuja G, Yuan D, Farre G, Arjo G, Berman J, Zorrilla U, Raviral B, Bai C, Pérez-Massot E, Bassie L, Capell T, Christou P (2012) Biofortification of plants with altered antioxidant content and composition: genetic engineering strategies. Plant Biotechnol J. doi: 10.1111/J.1467-7652.00740.x
Download references
Acknowledgments
Research at the Universitat de Lleida is supported by MICINN, Spain (BFU2007-61413); European Union Framework 7 Program-SmartCell Integrated Project 222716; European Union Framework 7 European Research Council IDEAS Advanced Grant Program-BIOFORCE; COST Action FA0804: Molecular farming: plants as a production platform for high value proteins; Centre CONSOLIDER on Agrigenomics funded by MICINN, Spain. All research programs in our laboratory are supported exclusively through public funds.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
Author information
Authors and affiliations.
University of Florence, Florence, Italy
Universitat de Lleida - Agrotecnio Center and ICREA, Barcelona, Spain
P. Christou
National Institute for Research on Food and Nutrition, Rome, Italy
You can also search for this author in PubMed Google Scholar
Corresponding authors
Correspondence to M. Buiatti , P. Christou or G. Pastore .
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Reprints and permissions
About this article
Cite this article.
Buiatti, M., Christou, P. & Pastore, G. The application of GMOs in agriculture and in food production for a better nutrition: two different scientific points of view. Genes Nutr 8 , 255–270 (2013). https://doi.org/10.1007/s12263-012-0316-4
Download citation
Received : 04 May 2012
Accepted : 03 August 2012
Published : 18 October 2012
Issue Date : May 2013
DOI : https://doi.org/10.1007/s12263-012-0316-4
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Genetically modified food
- Food production
- Agro food science
Genes & Nutrition
ISSN: 1865-3499
- General enquiries: [email protected]
Genetically Modified and Gene-Edited Food Crops: Recent Status and Future Prospects
- First Online: 02 April 2024
Cite this chapter

- Mousumi Sabat 23 &
- Ashutosh Tripathy 24
Part of the book series: Advances in Science, Technology & Innovation ((ASTI))
526 Accesses
Genetically modified (GM) and gene-edited (GE) food crops have emerged as transformative technologies in agriculture, offering potential solutions to address global food security challenges. This chapter overviews the current status and future prospects of genetically modified and gene-edited food crops, highlighting their existing applications and potential impacts on agriculture and society. Genetically modified crops involve intentionally changing an organism's genome by introducing foreign genes, which enable desired traits such as resistance to pests, diseases, or environmental stresses. Gene editing technologies, such as clustered regularly interspaced short palindromic repeats (CRISPR)-CRISPR-associated protein 9 (Cas9), transcription activator-like effector nucleases (TALENs), zinc-finger nucleases (ZFNs), etc. , have become a precise and efficient tool for targeted genetic modifications. Unlike traditional genetic modification, gene editing allows for precise alterations within an organism's genome without introducing foreign DNA. This technology has opened up new possibilities for crop improvement, including enhanced disease resistance, improved nutritional content, and increased tolerance to adverse growing conditions. Gene-edited crops hold significant promise for sustainable agriculture, with potential benefits including reduced chemical inputs, increased crop adaptability, and improved crop quality. The regulatory landscape surrounding genetically modified and gene-edited crops varies across countries, with some nations implementing strict regulations while others adopt a more permissive approach. Nevertheless, the future prospects of genetically modified and gene-edited food crops appear promising. Continued advancements in gene editing technologies, coupled with ongoing research on the safety and efficacy of these crops, will contribute to their further adoption and acceptance. However, careful monitoring of potential environmental impacts, addressing public concerns, and transparent communication about the benefits and risks of these technologies will be crucial to their successful integration into global agriculture.
This is a preview of subscription content, log in via an institution to check access.
Access this chapter
Subscribe and save.
- Get 10 units per month
- Download Article/Chapter or eBook
- 1 Unit = 1 Article or 1 Chapter
- Cancel anytime
- Available as PDF
- Read on any device
- Instant download
- Own it forever
- Available as EPUB and PDF
- Durable hardcover edition
- Dispatched in 3 to 5 business days
- Free shipping worldwide - see info
Tax calculation will be finalised at checkout
Purchases are for personal use only
Institutional subscriptions
Similar content being viewed by others
Genetically modified crop regulations: scope and opportunity using the CRISPR-Cas9 genome editing approach

Transgenic Strategies and Genome Editing for Nutritional Enrichment

Genome Editing Crops in Food and Futuristic Crops
Abbreviations.
Clustered Regularly Interspaced Short Palindromic Repeat-CRISPR Associated Protein 9
The Court of Justice of the European Union
2,4-Dichlorophenoxyacetic acid
Deoxyribonucleic Acid
United States Environmental Protection Agency
European Union
Food And Agriculture Organization
Food and Drug Administration
The Food Safety and Standards Authority of India
Granule-bound Starch Synthase I GM- Genetically Modified
Genetically Edited
The Genetic Engineering Appraisal Committee
Ministry of Agriculture and Rural Affairs
The Ministry of Agriculture & Farmers Welfare
Office of the Gene Technology Regulator
Polymerase Chain Reaction
The Review Committee on Genetic Manipulation
United State Department of Agriculture
World Health Organization
Abdallah NA, Prakash CS, McHughen AG (2015) Genome editing for crop improvement: challenges and opportunities. GM Crops & Food 6(4):183–205. https://doi.org/10.1080/21645698.2015.1129937
Article Google Scholar
Akshat M, Vijay V (2007) Successful commercialization of insect-resistant eggplant by a public-private partnership: reaching and benefiting resource-poor farmers. Intellectual property management in health and agricultural innovation: a handbook of best practices, Vol 1 and 2, pp 1829–1831
Google Scholar
Anderson JA, Ellsworth PC, Faria JC et al (2019) Genetically engineered crops: importance of diversified integrated pest management for agricultural sustainability. Front Bioeng Biotechnol 7:24. https://doi.org/10.3389/fbioe.2019.00024
Anderson JA, Gipmans M, Hurst S et al (2016) Emerging agricultural biotechnologies for sustainable agriculture and food security. J Agric Food Chem 64(2):383–393. https://doi.org/10.1021/acs.jafc.5b04543
Article CAS Google Scholar
Arora L, Narula A (2017) Gene editing and crop improvement using CRISPR-Cas9 system. Front Plant Sci 8:1932. https://doi.org/10.3389/fpls.2017.01932
Bawa AS, Anilakumar KR (2013) Genetically modified foods: safety, risks and public concerns—a review. J Food Sci Technol 50(6):1035–1046. https://doi.org/10.1007/s13197-012-0899-1
Bayer PE, Golicz AA, Scheben A et al (2020) Plant pan-genomes are the new reference. Nature Plants 6(8):914–920. https://doi.org/10.1038/s41477-020-0733-0
Boyle JH, Dalgleish HJ, Puzey JR (2019) Monarch butterfly and milkweed declines substantially predate the use of genetically modified crops. Proc Natl Acad Sci 116(8):3006–3011. https://doi.org/10.1073/pnas.1811437116
Brown Z (2016) Environmental release of engineered pests building an international governance framework. In Workshop Presentation at North Carolina State University Raleigh
Carpenter JE (2011) Impact of GM crops on biodiversity. GM Crops 2(1):7–23
CBD (2000) https://www.cbd.int/doc/legal/cartagena-protocol-en.pdf . Accessed 31 May 2023
Cheng H, Jin W, Wu H et al (2007) Isolation and PCR detection of foreign DNA sequences in bee honey raised on genetically modified bt (Cry 1 Ac) cotton. Food Bioprod Process 85(2):141–145. https://doi.org/10.1205/fbp06056
Cohen SN, Chang AC, Boyer HW et al (1973) Construction of biologically functional bacterial plasmids in vitro. Proc Natl Acad Sci 70(11):3240–3244. https://doi.org/10.1073/pnas.70.11.3240
Court of Justice of the European Union. Judgement of the Court in Case C-528/16: Court of Justice of the European Union. http://curia.europa.eu/juris/document/document.jsf?text=&docid=204387&pageIndex=0&doclang=EN&mode=lst&dir=&occ=first&part=1&cid=138460 . Accessed 23 May 2023
EFSA Panel on Genetically Modified Organisms (GMO) (2022) Scientific Opinion on development needs for the allergenicity and protein safety assessment of food and feed products derived from biotechnology. EFSA J 20(1):e07044. https://doi.org/10.2903/j.efsa.2022.7044
EFSA (2017) https://www.efsa.europa.eu/en/efsajournal/pub/5048 . Accessed 31 May 2013
EPA (2023) https://www.epa.gov/regulation-biotechnology-under-tsca-and-fifra/epas-regulation-biotechnology-use-pest-management . Accessed 31 May 2023
Eshed Y, Lippman ZB (2019) Revolutions in agriculture chart a course for targeted breeding of old and new crops. Science 366(6466). https://doi.org/10.1126/science.aax0025
FDA (2023) https://www.fda.gov/food/agricultural-biotechnology/how-gmos-are-regulated-united-states . Accessed 31 May 2023
Fernandes GB, Silva ACDL, Maronhas MES et al (2022) Transgene flow: Challenges to the on-farm conservation of maize landraces in the Brazilian semi-arid region. Plants 11(5):603. https://doi.org/10.3390/plants11050603
Fernandez-Cornejo J (2005) Adoption of genetically engineered crops in the US
Food and Agriculture Organization (2022) https://www.fao.org/3/cc3579en/cc3579en.pdf . Accessed 8 May 2023
GEAC (2017) http://geacindia.gov.in/about-geac-india.aspx . Accessed 31 May 2023
Giraldo PA, Shinozuka H, Spangenberg GC et al (2019) Safety assessment of genetically modified feed: is there any difference from food? Front Plant Sci 10:1592. https://doi.org/10.3389%2Ffpls.2019.01592
Gordon DR, Jaffe G, Doane M et al (2021) Responsible governance of gene editing in agriculture and the environment. Nat Biotechnol 39(9):1055–1057. https://doi.org/10.1038/s41587-021-01023-1
Hefferon KL (2015) Nutritionally enhanced food crops; progress and perspectives. Int J Mol Sci 16(2):3895–3914. https://doi.org/10.3390%2Fijms16023895
International Service for the Acquisition of Agri-biotech Applications (2019) ISAAA Brief 55–2019: Executive Summary Biotech Crops Drive Socio-Economic Development and Sustainable Environment in the New Frontier. https://www.isaaa.org/resources/publications/briefs/55/executivesummary/default.asp . Accessed 31 May 2023
James C (2011) Global status of commercialized biotech/GM crops, 2011 (Vol. 44). Ithaca, NY: isaaa
James C, Krattiger AF (1996) Global review of the field testing and commercialization of transgenic plants: 1986 to 1995. Isaaa Briefs, 1
Karembu M (2021) Genome Editing in Africa's Agriculture 2021: An Early Take-Off (ISAAA)
Koch MS, DeSesso JM, Williams AL et al (2016) Adaptation of the ToxRTool to assess the reliability of toxicology studies conducted with genetically modified crops and implications for future safety testing. Crit Rev Food Sci Nutr 56(3):512–526. https://doi.org/10.1080/10408398.2013.788994
Lau SE, Teo WFA, Teoh EY et al (2022) Microbiome engineering and plant biostimulants for sustainable crop improvement and mitigation of biotic and abiotic stresses. Discover Food 2(1):9. https://doi.org/10.1007/s44187-022-00009-5
Liang J, Yang X, Jiao Y et al (2022) The evolution of China’s regulation of agricultural biotechnology. Abiotech 1–13. https://doi.org/10.1007/s42994-022-00086-1
Liu G, Su W, Xu Q et al (2004) Liquid-phase hybridization based PCR-ELISA for detection of genetically modified organisms in food. Food Control 15(4):303–306. https://doi.org/10.1016/S0956-7135(03)00081-1
Lu K, Wu B, Wang J et al (2018) Blocking amino acid transporter Os AAP 3 improves grain yield by promoting outgrowth buds and increasing tiller number in rice. Plant Biotechnol J 16(10):1710–1722. https://doi.org/10.1111/pbi.12907
Maghari BM, Ardekani AM (2011) Genetically modified foods and social concerns. Avicenna J Med Biotechnol 3:109
McCabe MS, Power JB, de Laat AM et al (1997) Detection of single-copy genes in DNA from transgenic plants by nonradioactive Southern blot analysis. Mol Biotechnol 7:79–84. https://doi.org/10.1007/BF02821545
Midtvedt T (2014) Antibiotic resistance and genetically modified plants. Microb Ecol Health Dis 25(1):25918
Munawar S, ul Qamar MT, Mustafa G et al. (2020) Role of biotechnology in climate resilient agriculture. Environment, climate, plant and vegetation growth 339-365. https://doi.org/10.1007/978-3-030-49732-3_14
Nordlee JA, Taylor SL, Townsend JA et al (1996) Identification of a Brazil-nut allergen in transgenic soybeans. N Engl J Med 334(11):688–692
OGTR Gene Technology Act 2000 https://www.ogtr.gov.au/about-ogtr/australias-gene-technology-regulatory-system . Accessed 31 May 2023
Oliver MJ (2014) Why we need GMO crops in agriculture. Mo Med 111(6):492
Pan S, Qin B, Bi L (2021) An unsupervised learning method for the detection of genetically modified crops based on terahertz spectral data analysis. Secur Commun Netw 2021:1–7. https://doi.org/10.1155/2021/5516253
Pérez L, Soto E, Farré G et al (2019) CRISPR/Cas9 mutations in the rice Waxy/GBSSI gene induce allele-specific and zygosity-dependent feedback effects on endosperm starch biosynthesis. Plant Cell Rep 38:417–433. https://doi.org/10.1007/s00299-019-02388-z
Pixley KV, Falck-Zepeda JB, Paarlberg RL (2022) Genome-edited crops for improved food security of smallholder farmers. Nat Genet 54(4):364–367. https://doi.org/10.1038/s41588-022-01046-7
Quist D (2010) Vertical (trans) gene flow: implications for crop diversity and wild relatives. Biotechnl Biosaf Series 11
Redden R (2021) Genetic modification for agriculture—proposed revision of GMO regulation in Australia. Plants 10(4):747. https://doi.org/10.3390/plants10040747
Repellin A, Båga M, Jauhar PP et al (2001) Genetic enrichment of cereal crops via alien gene transfer: new challenges. Plant Cell, Tissue and Organ Cult 64:159–183. https://doi.org/10.1023/A:1010633510352
Roberts A, Boeckman CJ, Mühl M et al (2020) Sublethal endpoints in non-target organism testing for insect-active GE crops. Front Bioeng Biotechnol 8:556. https://doi.org/10.3389/fbioe.2020.00556
Schütte G, Eckerstorfer M, Rastelli V (2017) Herbicide resistance and biodiversity: agronomic and environmental aspects of genetically modified herbicide-resistant plants. Environ Sci Eur 29:1–12. https://doi.org/10.1186/s12302-016-0100-y
Sedeek KE, Mahas A, Mahfouz M (2019) Plant genome engineering for targeted improvement of crop traits. Front Plant Sci 10:114. https://doi.org/10.3389/fpls.2019.00114
Sharma P, Singh SP, Iqbal HM (2022) Genetic modifications associated with sustainability aspects for sustainable developments. Bioeng 13(4):9509–9521. https://doi.org/10.1080/21655979.2022.2061146
Steenwerth KL, Hodson AK, Bloom AJ et al (2014) Climate-smart agriculture global research agenda: scientific basis for action. Agric Food Secur 3(1):1–39. https://doi.org/10.1186/2048-7010-3-11
Touyz LZG (2013) Genetically modified foods, cancer, and diet: Myths and reality. Curr Oncol 20(2):59–61
US Department of Agriculture (2018) Secretary Perdue Issues USDA Statement on Plant Breeding Innovation. Washington, DC. https://www.usda.gov/media/press-releases/2018/03/28/secretary-perdue-issues-usda-statement-plant-breeding-innovation . Accessed 23 May 2023
USDA (2023) https://www.usda.gov/topics/biotechnology/how-federal-government-regulates-biotech-plants . Accessed 31 May 2023
Van Der Straeten D, Bhullar NK, De Steur H (2020) Multiplying the efficiency and impact of biofortification through metabolic engineering. Nat Commun 11(1):5203. https://doi.org/10.1038/s41467-020-19020-4
Van Esse HP, Reuber TL, Van der Does D (2020) Genetic modification to improve disease resistance in crops. New Phytol 225(1):70–86. https://doi.org/10.1111/nph.15967
Vieira LR, Freitas NC, Justen F et al. (2021) Regulatory framework of genome editing in Brazil and worldwide. https://www.alice.cnptia.embrapa.br/bitstream/doc/1132164/1/Regulatory-framework-of-genome-CAP-5.pdf
Wang T, Zhang H, Zhu H (2019) CRISPR technology is revolutionizing the improvement of tomato and other fruit crops. Hortic Res 6. https://doi.org/10.1038/s41438-019-0159-x
World Health Organization (2014) “Frequently asked questions on genetically modified foods”. Retrieved 9 May 2023
Xiao X, Wu H, Zhou X et al (2012) The combination of quantitative PCR and western blot detecting CP4-EPSPS component in Roundup Ready soy plant tissues and commercial soy-related foodstuffs. J Food Sci 77(6):C603–C608. https://doi.org/10.1111/j.1750-3841.2012.02718.x
Xiao Z, Kerr WA (2022) Biotechnology in China–regulation, investment, and delayed commercialization. GM Crops & Food 13(1):86–96. https://doi.org/10.1080/21645698.2022.2068336
Ye R, Yang X, Rao Y (2022) Genetic engineering technologies for improving crop yield and quality. Agron 12(4):759. https://doi.org/10.3390/agronomy12040759
Ye X, Al-Babili S, Kloti A et al (2000) Engineering the provitamin A (β-carotene) biosynthetic pathway into (carotenoid-free) rice endosperm. Science 287(5451):303–305. https://doi.org/10.1126/science.287.5451.303
Zhang S, Zhang R, Gao J (2021) CRISPR/Cas9-mediated genome editing for wheat grain quality improvement. Plant Biotechnol J 19(9):1684. https://doi.org/10.1111%2Fpbi.13647
Zilberman D, Sexton SE, Marra M et al (2010) The economic impact of genetically engineered crops. Choices 25(2)
Zohary D, Hopf M, Weiss E (2012) Domestication of plants in the old world: the origin and spread of domesticated plants in Southwest Asia, Europe, and the Mediterranean Basin. Oxford University Press
Book Google Scholar
Download references
Author information
Authors and affiliations.
ICAR—Central Institute of Agricultural Engineering, Bhopal, India
Mousumi Sabat
Institute of Chemical Technology, Mumbai, India
Ashutosh Tripathy
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Mousumi Sabat .
Editor information
Editors and affiliations.
Department of Botany, Acharya Prafulla Chandra Roy Government College, Matigara, Siliguri, West Bengal, India
Rakhi Chakraborty
Department of Botany, University of North Bengal, Raja Rammohunpur, Siliguri, West Bengal, India
Piyush Mathur
Swarnendu Roy
Rights and permissions
Reprints and permissions
Copyright information
© 2024 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Sabat, M., Tripathy, A. (2024). Genetically Modified and Gene-Edited Food Crops: Recent Status and Future Prospects. In: Chakraborty, R., Mathur, P., Roy, S. (eds) Food Production, Diversity, and Safety Under Climate Change. Advances in Science, Technology & Innovation. Springer, Cham. https://doi.org/10.1007/978-3-031-51647-4_18
Download citation
DOI : https://doi.org/10.1007/978-3-031-51647-4_18
Published : 02 April 2024
Publisher Name : Springer, Cham
Print ISBN : 978-3-031-51646-7
Online ISBN : 978-3-031-51647-4
eBook Packages : Earth and Environmental Science Earth and Environmental Science (R0)
Share this chapter
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Publish with us
Policies and ethics
- Find a journal
- Track your research

IMAGES
VIDEO
COMMENTS
Technologies for genetically modifying foods offer dramatic promise for meeting some areas of greatest challenge for the 21st century. Like all new technologies, they also pose some risks, both known and unknown.
In this paper, we attempt to summarize up-to-date knowledge about the benefits and potential problems of GM food. We also introduce some recent technological developments in GM foods and their impact in the field.
A systematic review of animal and human studies was conducted on genetically modified (GM) food consumption to assess its safety in terms of adverse effects/events to inform public concerns and future research.
Genetically modified (GM) foods have revolutionized agricultural biotechnology since their debut in the mid-1990s, sparking debates about their safety, environmental impact, and potential to...
Genetic modification in plants was first recorded 10,000 years ago in Southwest Asia where humans first bred plants through artificial selection and selective breeding. Since then, advancements in agriculture science and technology have brought about the current GM crop revolution.
In this applied study using data from 2018 to 2023 and a sample of 3101 respondents from the U.S. state of Vermont, we analyze changes in support for a variety of GM attributes over time.
Controversies and public concern surrounding GM foods and crops commonly focus on human and environmental safety, labelling and consumer choice, intellectual property rights, ethics, food security, poverty reduction and environmental conservation.
Consumer attitudes with respect to genetically modified foods differ widely, particularly between North America and Europe. Information asymmetry, incomplete information and uncertainty arise as a result of concerns over GMOs.
Genetically modified (GM) and gene-edited (GE) food crops have emerged as transformative technologies in agriculture, offering potential solutions to address global food security challenges. This chapter overviews the current status and future prospects of...
GMF scientists have promised genetically modified food to have a higher nutritional value, better texture, longer lifespan, virus resistance, and better flavor (Gatew et al. 2019). ...