
- Science Notes Posts
- Contact Science Notes
- Todd Helmenstine Biography
- Anne Helmenstine Biography
- Free Printable Periodic Tables (PDF and PNG)
- Periodic Table Wallpapers
- Interactive Periodic Table
- Periodic Table Posters
- Science Experiments for Kids
- How to Grow Crystals
- Chemistry Projects
- Fire and Flames Projects
- Holiday Science
- Chemistry Problems With Answers
- Physics Problems
- Unit Conversion Example Problems
- Chemistry Worksheets
- Biology Worksheets
- Periodic Table Worksheets
- Physical Science Worksheets
- Science Lab Worksheets
- My Amazon Books

Scientific Method Vocabulary Terms

The scientific method involves a hypothesis, variables, controls, experiments, and other concepts and terms that may be confusing. This is a glossary of key scientific method vocabulary terms and their definitions .
Glossary of Scientific Method Words
Anomaly: An anomaly is an observation that differs from expectation or from accepted scientific views. Anomalies lead scientists to revise a hypothesis or theory.
Central Limit Theorem: The central limit theorem states that with a sufficiently large sample, the sample mean will be normally distributed. A normally distributed sample mean is necessary to apply the t test, so if you are planning to perform a statistical analysis of experimental data, it’s important to have a big sample.
Conclusion: The conclusion is your determination of whether the hypothesis should be accepted or rejected. It is one of the steps of the scientific method.
Control Group : The control group is the set of test subjects randomly assigned to not receive the experimental treatment. In other words, the independent variable is held constant for this group.
Control Variable : A control is any variable that does not change during an experiment. It is also known as a constant variable.
Correlation: A correlation is a relationship between two variables that can be used to predict the behavior or value of one variable if the other is known. Correlation is not the same as causality. In other words, correlating two variables doesn’t always imply one causes the other.
Data: (singular: datum) Data refers to any facts, numbers, or values obtained in an experiment.
Data Table: This is a T-shaped diagram used to display data from a science experiment. It includes the values of the independent and dependent variables.
Dependent Variable: The dependent variable is the variable that responds to the independent variable. It is the one that is measured in the experiment. It is also known as the dependent measure , responding variable.
Double-blind : When an experiment is double-blind, it means neither the researcher nor the subject knows whether the subject is receiving the treatment or a placebo. “Blinding” helps reduce biased results.
Empty Control Group: An empty control group is a type of control group which does not receive any treatment, including a placebo.
Error : Error is a measure of the difference between a measured or calculated value and a true value.
Experiment : An experiment is a procedure that tests a hypothesis.
Experimental Group: The experimental group is the set of test subjects randomly assigned to receive the experimental treatment.
Extraneous Variable: Extraneous variables are extra variables (i.e., not the independent, dependent, or control variables) that may influence an experiment, but are not accounted for or measured or are beyond control. Examples may include factors you consider unimportant at the time of an experiment, such as the manufacturer of the glassware in a reaction or the color of paper used to make a paper airplane.
Fact: A fact is a statement based on evidence obtained from direct observation.
Graph: A graph is a picture that displays information. Examples of graphs include line graphs and bar graphs. The most common type of graph displays values of the independent and dependent variables.
Hypothesis: A hypothesis is a prediction of whether the independent variable will have an effect on the dependent variable or a prediction of the nature of the effect.
Independence or Independently: Independence means one factor does not exert influence on another. For example, what one study participant does should not influence what another participant does. They make decisions independently. Independence is critical for a meaningful statistical analysis.
Independent Random Assignment: Independent random assignments means randomly selecting whether a test subject will be in a treatment or control group.
Independent Variable: The independent variable is the variable that is manipulated or changed by the researcher. There is one independent variable in an experiment.
Independent Variable Levels: Independent variable levels refers to changing the independent variable from one value to another (e.g., different drug doses, different time duration). The different values are called “levels.”
Inferential Statistics: Inferential statistics means applying statistics (math) to infer characteristics of a population based on a representative sample from the population.
Internal Validity: An experiment is said to have internal validity if it can accurately determine whether the independent variable produces an effect.
Law : A scientific law is a generalization that describes what one expects to happen in a certain situation. For example, the law of gravity makes it possible to predict an object will fall if it is dropped. Laws can be used to predict behavior, but do not explain it.
Log Book: A log book or notebook records all of a scientist’s observations about an experiment. Entries are typically recorded in permanent ink.
Mean: The mean is the average calculated by adding up all the scores and then dividing by the number of scores.
Null Hypothesis : Th null hypothesis is the “no difference” or “no effect” hypothesis, which predicts the treatment will not have an effect on the subject. The null hypothesis is easier to assess with a statistical analysis than other forms of a hypothesis.
Null Results (Nonsignificant Results): If a researcher obtains nulls results, it means the results do not disprove the null hypothesis. Null results don’t prove the null hypothesis, because the results may have resulted from a lack of power. Some null results are type 2 errors.
Observation: An observation is information collected using one of the senses (sight, sound, touch, taste, scent).
p < 0.05: This is an indication of how often chance alone could account for the effect of the experimental treatment. A value p < 0.05 means that 5 times out of a hundred, you could expect this difference between the two groups, purely by chance. Since the chance of the effect occurring by chance is so small, the researcher may conclude the experimental treatment did indeed have an effect. Note other p or probability values are possible. The 0.05 or 5% limit simply is a common benchmark of statistical significance.
Placebo (Placebo Treatment): A placebo is a fake treatment that should have no effect, outside of the power of suggestion. Example: In drug trials, test patients may be given a pill containing the drug or a placebo, which resembles the drug (pill, injection, liquid) but doesn’t contain the active ingredient.
Placebo Effect : The placebo effect is a beneficial effect due to a subject’s belief in the power of the treatment. No active ingredient or other property of the placebo is responsible for the positive effect.
Population: A population is the entire group the researcher is studying. If the researcher cannot gather data from the population, studying large random samples taken from the population may be used to estimate how the population would respond.
Power: Power reflects the ability to observe differences or avoid making Type 2 errors .
Random or Randomness: To be random means to be selected or performed without following any pattern or method. To avoid unintentional bias, researchers often use random number generators or flip coins to make selections. (learn more)
Results: The results are the explanation or interpretation of experimental data. This includes calculations made from the data.
Statistical Significance: Statistical significance is the observation, based on the application of a statistical test, that a relationship probably is not due to pure chance. The probability is stated (e.g., p < 0.05) and the results are said to be statistically significant .
Simple Experiment : A simple experiment is a basic experiment designed to assess whether there are a cause and effect relationship or test a prediction. A fundamental simple experiment may have only one test subject, compared with a controlled experiment, which has at least two groups.
Single-blind: A single-blind conditions occurs when either the experimenter or subject is unaware whether the subject is getting the treatment or a placebo. Blinding the researcher helps prevent bias when the results are analyzed. Blinding the subject prevents the participant from having a biased reaction.
T-test: The T-test is a common statistical data analysis applied to experimental data to test a hypothesis. The t-test computes the ratio between the difference between the group means and the standard error of the difference (a measure of the likelihood the group means could differ purely by chance). A rule of thumb is that the results are statistically significant if you observe a difference between the values that are three times larger than the standard error of the difference, but it’s best to look up the ratio required for significance on a t table.
Theory : A theory is a systematic explanation for phenomena, based on testing many hypotheses. Because they are evidence-based, theories are typically accepted by scientists, but they may be modified or discarded if new evidence is presented.
Type I Error (Type 1 error): A type I error occurs when you reject the null hypothesis, but it was actually true. If you perform the t-test and set p < 0.05, there is less than a 5% chance you could make a Type I error by rejecting the hypothesis based on random fluctuations in the data.
Type II Error (Type 2 error): A type II error occurs when you accept the null hypothesis, but it was actually false. The experimental conditions had an effect, but the researcher failed to find it statistically significant.
Further Study
Test your understanding of the scientific method glossary by taking a brief scientific method quiz . Solve a scientific method word search puzzle to gain familiarity with the terms.
Related Posts

Chemistry Dictionary/Terms/Glossary/Word List A to Z
- December 8, 2021
The chemistry dictionary offers definitions and examples of important chemistry and chemical-related terms, which are listed in alphabetical order. For each term, a brief definition is given. Each link leads to a more comprehensive discussion of the word. Additional definitions are also available.
Chemistry Terms Starting with A to Z
Share this to:
You may also like to read:

Banana peels: A waste treasure for Humans
Detection of Carbohydrates and glycosides: Detection of functional group

How to read IR spectra: 7 easy steps
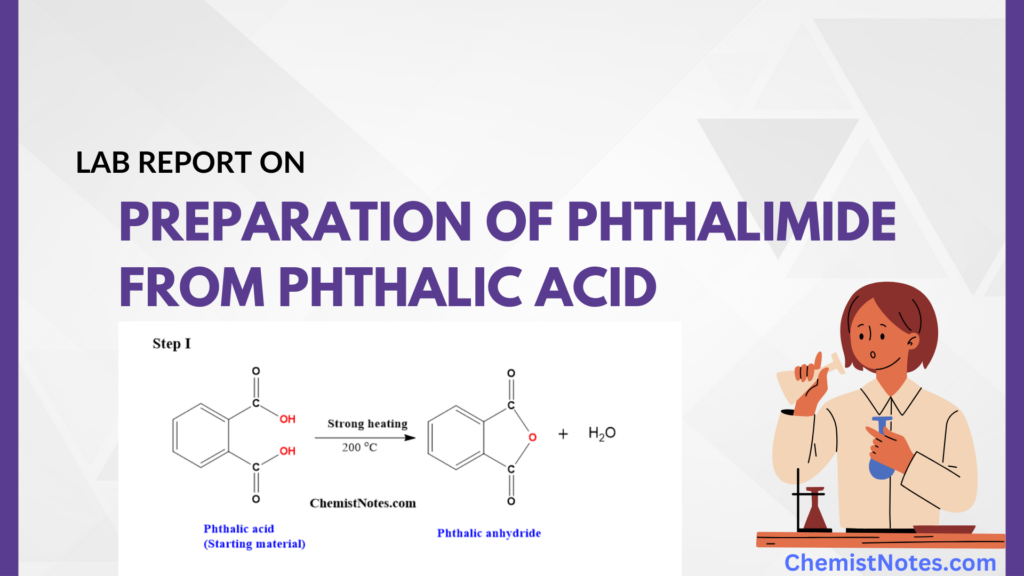
Preparation of Phthalimide from Phthalic acid by two-step synthesis: Useful Lab Report

Feel Good Hormones: Dopamine, Oxytocin, Serotonin, and Endorphin

Invisible ink: Chemistry, Properties, and 3 Reliable Application

Pyrrole Disorder: Symptoms, Causes, and Treatment

The Chemistry of Mehendi: Composition, Side effects, and Reliable Application

How to Balance Redox Equations Using a Redox Reaction Calculator
Leave a Reply Cancel reply
Your email address will not be published. Required fields are marked *
Save my name, email, and website in this browser for the next time I comment.
Top Universities in the USA for Undergraduate and Graduate Chemistry Courses
Sugar Vs Jaggery: Differences, Calories And Many more
Centrifugation: Definition, Principle, Types, and 3 Reliable Application
Eppendorf Tube: Definition, Types, and Reliable Uses
Litmus Paper: Definition, Chemistry, Test, and 4 important Applications
Micropipette: Definition, Types, and 8 Reliable Application
Rotary Evaporator: Introduction, working principle, application, and Price
Analytical Balance: Definition, Principle, and Reliable uses
Nutrient Agar: Definition, Composition, and Reliable uses
Microplate washer: Principle, Working, And Reliable uses
96 well plate: Definition, Types, Reliable uses
Our mission is to provide free, world-class Chemisry Notes to Students, anywhere in the world.
Chemist Notes | Chemistry Notes for All | 2024 - Copyright©️ ChemistNotes.com
Chemistry-Dictionary.com
Everyone's Online Chemistry Dictionary
Chemistry Dictionary
Thank you for visiting! Our website is a free online chemistry dictionary containing over 1,800 chemistry terms and definitions.
We invite Everyone to use our dictionary of chemistry terminology to help further their knowledge, students and teachers alike. To start browsing the dictionary, choose a letter to list all the terms starting with that letter.
You can search our dictionary using the search box provided.
Our collection of chemistry terms and definitions cover all areas, including: organic chemistry, inorganic, biological, physical, and analytical chemistry.
Students of all study levels use our site to find solutions and help for their problems, questions and projects.
—–
Whether a chemistry student, professional, or simply curious, our Chemistry Dictionary is a valuable resource for everyone.
We strive to provide clear, concise, and accurate definitions for all things chemistry, along with supplementary information and examples to help you better understand the concepts at hand.
Our goal is to offer clear, concise, and accurate definitions in the field of chemistry. We supplement these with extra information and examples to aid comprehension of the concepts.
What is Chemistry?
Before exploring our comprehensive collection of terms, definitions, and resources, let’s address the basic query: What is chemistry?
Chemistry is the scientific study of matter, its properties, and the changes it undergoes. Fundamentally, chemistry aims to understand how atoms, molecules, and compounds – the world’s building blocks – interact and transform. Chemistry is often termed the “central science”. It links physics, biology, and other natural sciences. This connection allows for enhanced understanding and manipulation at the molecular level.
Chemistry is a diverse and multifacete field, with various branches such as organic, inorganic, physical, analytical, and biochemistry. Each branch explores the complex details of chemical reactions and processes. These insights contribute to a wide array of fields. They aid in creating new materials and medicines, understanding biological mechanisms, and investigating environmental phenomena.
Types of Chemistry
Organic chemistry.
Organic chemistry is the branch of chemistry focusing on the study of carbon-containing compounds, their structures, properties, synthesis, and reactions. The unique capability of carbon to forge strong bonds with other atoms, particularly itself, leads to a plethora of diverse organic molecules. These molecules display varied properties and functions.
This field plays a crucial role in in understanding life’s chemistry. It includes studying biomolecules like carbohydrates, proteins, lipids, and nucleic acids. Additionally, organic chemistry is integral to the development of new materials, pharmaceuticals, and agrochemicals, making it a vital area of research and innovation.
Biochemistry
Biochemistry is the branch of chemistry that investigates the chemical processes and substances occurring within living organisms. By exploring the complex molecular interactions in biological systems, biochemists seek to understand the underlying mechanisms of cellular function, growth, development, and disease.
This interdisciplinary field combines elements of biology, chemistry, and physics to study the structures and functions of biomolecules such as proteins, nucleic acids, lipids, and carbohydrates, and their roles in metabolic pathways, genetic information transfer, and cellular signaling. Biochemistry has far-reaching applications in areas such as medicine, genetics, nutrition, and environmental science.
Inorganic Chemistry
Inorganic chemistry is the branch of chemistry that deals with the study of elements and their compounds, excluding those based on carbon-hydrogen bonds (which fall under organic). This vast field encompasses the entire periodic table and includes the study of metals, non-metals, and semi-metals, as well as coordination chemistry, solid-state chemistry, and organometallic chemistry.
Inorganic chemistry plays a significant role in the development of advanced materials, catalysis, and energy production, with applications ranging from electronics and superconductors to pigments, coatings, and fertilizers.
Analytical Chemistry
Analytical chemistry is the branch of chemistry concerned with the qualitative and quantitative determination of chemical components in various materials, substances, and environments. This field involves the development, optimization, and application of analytical methods and instrumentation to accurately measure, identify, and characterize chemical species.
Also vital in numerous industries, including pharmaceuticals, food safety, forensics, environmental monitoring, and quality control. Common analytical techniques include spectroscopy, chromatography, mass spectrometry, and electrochemistry, among others.
Physical Chemistry
Physical chemistry is the branch of chemistry that investigates the underlying principles governing chemical phenomena by applying the concepts and methods of physics. This field seeks to understand the fundamental behaviors and properties of molecules and chemical systems at the atomic and molecular levels.
Physical chemistry is divided into several subdisciplines, such as thermodynamics, quantum chemistry, kinetics, and statistical mechanics. By exploring the relationship between energy, structure, and reactivity, physical chemists contribute to diverse areas of research and application, including materials science, surface science, and molecular modeling.
Chemistry Dictionary Questions & Answers
What is chemistry-dictionary.com.
Chemistry-Dictionary.com is an online dictionary that provides definitions and explanations of chemistry-related terms and concepts. It serves as a reference for students, teachers, and professionals in the field of chemistry.
What kind of terms are included in the chemistry dictionary?
The chemistry dictionary includes terms and definitions used in chemistry. It covers subjects such as organic and inorganic chemistry, biochemistry, physical chemistry, analytical chemistry, and environmental chemistry.

68 Best Chemistry Experiments: Learn About Chemical Reactions
Whether you’re a student eager to explore the wonders of chemical reactions or a teacher seeking to inspire and engage your students, we’ve compiled a curated list of the top 68 chemistry experiments so you can learn about chemical reactions.
While the theories and laws governing chemistry can sometimes feel abstract, experiments bridge the gap between these concepts and their tangible manifestations. These experiments provide hands-on experiences illuminating the intricacies of chemical reactions, molecular structures, and elemental properties.
1. Covalent Bonds

By engaging in activities that demonstrate the formation and properties of covalent bonds, students can grasp the significance of these bonds in holding atoms together and shaping the world around us.
Learn more: Covalent Bonds
2. Sulfuric Acid and Sugar Demonstration
Through this experiment, students can develop a deeper understanding of chemical properties, appreciate the power of chemical reactions, and ignite their passion for scientific exploration.
3. Make Hot Ice at Home
Making hot ice at home is a fascinating chemistry experiment that allows students to witness the captivating transformation of a liquid into a solid with a surprising twist.
4. Make a Bouncing Polymer Ball

This hands-on activity not only allows students to explore the fascinating properties of polymers but also encourages experimentation and creativity.
Learn more: Thought Co
5. Diffusion Watercolor Art

This experiment offers a wonderful opportunity for students to explore the properties of pigments, observe how they interact with water, and discover the mesmerizing patterns and textures that emerge.
Learn more: Diffusion Watercolor Art
6. Exploding Baggie

The exploding baggie experiment is a captivating and dynamic demonstration that students should engage in with caution and under the supervision of a qualified instructor.
Learn more: Exploding Baggie
7. Color Changing Chemistry Clock

This experiment not only engages students in the world of chemical kinetics but also introduces them to the concept of a chemical clock, where the color change acts as a timekeeping mechanism.
Learn more: Color Changing Chemistry Clock
8. Pipe Cleaner Crystal Trees

By adjusting the concentration of the Borax solution or experimenting with different pipe cleaner arrangements, students can customize their crystal trees and observe how it affects the growth patterns.
Learn more: Pipe Cleaner Crystal Trees
9. How To Make Ice Sculptures

Through this experiment, students gain a deeper understanding of the physical and chemical changes that occur when water freezes and melts.
Learn more: Ice Sculpture
10. How to Make Paper

Through this hands-on activity, students gain a deeper understanding of the properties of cellulose fibers and the transformative power of chemical reactions.
Learn more: How to Make Paper
11. Color Changing Chemistry
Color changing chemistry is an enchanting experiment that offers a captivating blend of science and art. Students should embark on this colorful journey to witness the mesmerizing transformations of chemicals and explore the principles of chemical reactions.
12. Gassy Banana
The gassy banana experiment is a fun and interactive way for students to explore the principles of chemical reactions and gas production.
Learn more: Gassy Banana
13. Gingerbread Man Chemistry Experiment

This hands-on activity not only introduces students to the concepts of chemical leavening and heat-induced reactions but also allows for creativity in decorating and personalizing their gingerbread creations.
Learn more: Gingerbread Man Chemistry Experiment
14. Make Amortentia Potion

While the love potion is fictional, this activity offers a chance to explore the art of potion-making and the chemistry behind it.
Learn more: How to Make Amortentia Potion
15. Strawberry DNA Extraction
This hands-on experiment offers a unique opportunity to observe DNA, the building blocks of life, up close and learn about its structure and properties.
16. Melting Snowman

The melting snowman experiment is a fun and whimsical activity that allows students to explore the principles of heat transfer and phase changes.
Learn more: Melting Snowman
17. Acid Base Cabbage Juice

The acid-base cabbage juice experiment is an engaging and colorful activity that allows students to explore the pH scale and the properties of acids and bases.
By extracting the purple pigment from red cabbage leaves and creating cabbage juice, students can use this natural indicator to identify and differentiate between acidic and basic substances.
Learn more: Acid Base Cabbage Juice
18. Magic Milk

The magic milk experiment is a mesmerizing and educational activity that allows students to explore the concepts of surface tension and chemical reactions.
By adding drops of different food colors to a dish of milk and then introducing a small amount of dish soap, students can witness a captivating display of swirling colors and patterns.
Learn more: Magic Milk
19. Melting Ice with Salt and Water

Through this hands-on activity, students can gain a deeper understanding of the science behind de-icing and how different substances can influence the physical properties of water.
Learn more: Melting Ice with Salt and Water
20. Barking Dog Chemistry Demonstration

The barking dog chemistry demonstration is an exciting and visually captivating experiment that showcases the principles of combustion and gas production.
21. How to Make Egg Geodes

Making egg geodes is a fascinating and creative chemistry experiment that students should try. By using common materials like eggshells, salt, and food coloring, students can create their own beautiful geode-like crystals.
Learn more: How to Make Egg Geodes
22. Make Sherbet

This experiment not only engages the taste buds but also introduces concepts of acidity, solubility, and the chemical reactions that occur when the sherbet comes into contact with moisture.
Learn more: Make Sherbet
23. Hatch a Baking Soda Dinosaur Egg

As the baking soda dries and hardens around the toy, it forms a “shell” resembling a dinosaur egg. To hatch the egg, students can pour vinegar onto the shell, causing a chemical reaction that produces carbon dioxide gas.
Learn more: Steam Powered Family
24. Chromatography Flowers

By analyzing the resulting patterns, students can gain insights into the different pigments present in flowers and the science behind their colors.
Learn more: Chromatography Flowers
25. Turn Juice Into Solid

Turning juice into a solid through gelification is an engaging and educational chemistry experiment that students should try. By exploring the transformation of a liquid into a solid, students can gain insights of chemical reactions and molecular interactions.
Learn more: Turn Juice into Solid
26. Bouncy Balls
Making bouncy balls allows students to explore the fascinating properties of polymers, such as their ability to stretch and rebound.
27. Make a Lemon Battery
Creating a lemon battery is a captivating and hands-on experiment that allows students to explore the fundamentals of electricity and chemical reactions.
28. Mentos and Soda Project
The Mentos and soda project is a thrilling and explosive experiment that students should try. By dropping Mentos candies into a bottle of carbonated soda, an exciting eruption occurs.
29. Alkali Metal in Water
The reaction of alkali metals with water is a fascinating and visually captivating chemistry demonstration.
30. Rainbow Flame
The rainbow flame experiment is a captivating and visually stunning chemistry demonstration that students should explore.
31. Sugar Yeast Experiment
This experiment not only introduces students to the concept of fermentation but also allows them to witness the effects of a living organism, yeast, on the sugar substrate.
32. The Thermite Reaction
The thermite reaction is a highly energetic and visually striking chemical reaction that students can explore with caution and under proper supervision.
This experiment showcases the principles of exothermic reactions, oxidation-reduction, and the high temperatures that can be achieved through chemical reactions.
33. Polishing Pennies
Polishing pennies is a simple and enjoyable chemistry experiment that allows students to explore the concepts of oxidation and cleaning methods.
34. Elephant Toothpaste
The elephant toothpaste experiment is a thrilling and visually captivating chemistry demonstration that students should try with caution and under the guidance of a knowledgeable instructor.
35. Magic Potion
Creating a magic potion is an exciting and imaginative activity that allows students to explore their creativity while learning about the principles of chemistry.

36. Color Changing Acid-Base Experiment

Through the color changing acid-base experiment, students can gain a deeper understanding of chemical reactions and the role of pH in our daily lives.
Learn more: Color Changing Acid-Base Experiment
37. Fill up a Balloon
Filling up a balloon is a simple and enjoyable physics experiment that demonstrates the properties of air pressure. By blowing air into a balloon, you can observe how the balloon expands and becomes inflated.
38. Jello and Vinegar

The combination of Jello and vinegar is a fascinating and tasty chemistry experiment that demonstrates the effects of acid on a gelatin-based substance.
Learn more: Jello and Vinegar
39. Vinegar and Steel Wool Reaction

This experiment not only provides a visual demonstration of the oxidation process but also introduces students to the concept of corrosion and the role of acids in accelerating the process.
Learn more: Vinegar and Steel Wool Reaction
40. Dancing Rice

The dancing rice experiment is a captivating and educational demonstration that showcases the principles of density and buoyancy.
By pouring a small amount of uncooked rice into a clear container filled with water, students can witness the rice grains moving and “dancing” in the water.
Learn more: Dancing Rice
41. Soil Testing Garden Science

Soil testing is a valuable and informative experiment that allows students to assess the composition and properties of soil.
By collecting soil samples from different locations and analyzing them, students can gain insights into the nutrient content, pH level, and texture of the soil.
Learn more: Soil Testing Garden Science
42. Heat Sensitive Color Changing Slime

Creating heat-sensitive color-changing slime is a captivating and playful chemistry experiment that students should try.
Learn more: Left Brain Craft Brain
43. Experimenting with Viscosity

Experimenting with viscosity is an engaging and hands-on activity that allows students to explore the flow properties of liquids.
Viscosity refers to a liquid’s resistance to flow, and this experiment enables students to investigate how different factors affect viscosity.
Learn more: Experimenting with Viscosity
44. Rock Candy Science

Rock candy science is a delightful and educational chemistry experiment that students should try. By growing their own rock candy crystals, students can learn about crystal formation and explore the principles of solubility and saturation.
Learn more: Rock Candy Science
45. Baking Soda vs Baking Powder

Baking soda and baking powder have distinct properties that influence the leavening process in different ways.
This hands-on experiment provides a practical understanding of how these ingredients interact with acids and moisture to create carbon dioxide gas.
46. Endothermic and Exothermic Reactions Experiment

The endothermic and exothermic reactions experiment is an exciting and informative chemistry exploration that students should try.
By observing and comparing the heat changes in different reactions, students can gain a deeper understanding of energy transfer and the concepts of endothermic and exothermic processes.
Learn more: Education.com
47. Diaper Chemistry

By dissecting a diaper and examining its components, students can uncover the chemical processes that make diapers so effective at absorbing and retaining liquids.
Learn more: Diaper Chemistry
48. Candle Chemical Reaction
The “Flame out” experiment is an intriguing and educational chemistry demonstration that students should try. By exploring the effects of a chemical reaction on a burning candle, students can witness the captivating moment when the flame is extinguished.
49. Make Curds and Whey

This experiment not only introduces students to the concept of acid-base reactions but also offers an opportunity to explore the science behind cheese-making.
Learn more: Tinkerlab
50. Grow Crystals Overnight

By creating a supersaturated solution using substances like epsom salt, sugar, or borax, students can observe the fascinating process of crystal growth. This experiment allows students to explore the principles of solubility, saturation, and nucleation.
Learn more: Grow Crystals Overnight
51. Measure Electrolytes in Sports Drinks
The “Measure Electrolytes in Sports Drinks” experiment is an informative and practical chemistry activity that students should try.
By using simple tools like a multimeter or conductivity probe, students can measure the electrical conductivity of different sports drinks to determine their electrolyte content.
52. Oxygen and Fire Experiment
The oxygen and fire experiment is a captivating and educational chemistry demonstration that students should try. By observing the effects of oxygen on a controlled fire, students can witness the essential role of oxygen in supporting combustion.
53. Electrolysis Of Water
The electrolysis of water experiment is a captivating and educational chemistry demonstration that students should try.
Learn more: Electrolysis Of Water
54. Expanding Ivory Soap

The expanding Ivory Soap experiment is a fun and interactive chemistry activity that students should try. By placing a bar of Ivory soap in a microwave, students can witness the remarkable expansion of the soap as it heats up.
Learn more: Little Bins Little Hands
55. Glowing Fireworks

This experiment not only introduces students to the principles of pyrotechnics and combustion but also encourages observation, critical thinking, and an appreciation for the physics and chemistry behind.
Learn more: Glowing Fireworks
56. Colorful Polymer Chemistry

Colorful polymer chemistry is an exciting and vibrant experiment that students should try to explore polymers and colorants.
By combining different types of polymers with various colorants, such as food coloring or pigments, students can create a kaleidoscope of colors in their polymer creations.
Learn more: Colorful Polymer Chemistry
57. Sulfur Hexafluoride- Deep Voice Gas
This experiment provides a firsthand experience of how the density and composition of gases can influence sound transmission.
It encourages scientific curiosity, observation, and a sense of wonder as students witness the surprising transformation of their voices.
58. Liquid Nitrogen Ice Cream

Liquid nitrogen ice cream is a thrilling and delicious chemistry experiment that students should try. By combining cream, sugar, and flavorings with liquid nitrogen, students can create ice cream with a unique and creamy texture.
59. White Smoke Chemistry Demonstration

The White Smoke Chemistry Demonstration provides an engaging and visually captivating experience for students to explore chemical reactions and gases. By combining hydrochloric acid and ammonia solutions, students can witness the mesmerizing formation of white smoke.
60. Nitrogen Triiodide Chemistry Demonstration

The nitrogen triiodide chemistry demonstration is a remarkable and attention-grabbing experiment that students should try under the guidance of a knowledgeable instructor.
By reacting iodine crystals with concentrated ammonia, students can precipitate nitrogen triiodide (NI3), a highly sensitive compound.
61. Make a Plastic- Milk And Vinegar Reaction Experiment

Through the “Make a Plastic – Milk and Vinegar Reaction” experiment, students can gain a deeper understanding of the chemistry behind plastics, environmental sustainability, and the potential of biodegradable materials.
Learn more: Rookie Parenting
62. Eno and Water Experiment
This experiment not only introduces students to acid-base reactions but also engages their senses as they witness the visible and audible effects of the reaction.
63. The Eternal Kettle Experiment
By filling a kettle with alcohol and igniting it, students can investigate the behavior of the alcohol flame and its sustainability.
64. Coke and Chlorine Bombs
Engaging in this experiment allows students to experience the wonders of chemistry firsthand, making it an ideal choice to ignite their curiosity and passion for scientific exploration.
65. Set your Hand on Fire
This experiment showcases the fascinating nature of combustion and the science behind fire.
By carefully following proper procedures and safety guidelines, students can witness firsthand how the sanitizer’s high alcohol content interacts with an open flame, resulting in a brief but captivating display of controlled combustion.
66. Instant Ice Experiments
The Instant Ice Experiment offers an engaging and captivating opportunity for students to explore the wonders of chemistry and phase changes.
By using simple household ingredients, students can witness the fascinating phenomenon of rapid ice formation in just a matter of seconds.
67. Coke Cans in Acid and Base
Engaging in this experiment allows students to gain a deeper understanding of the chemical properties of substances and the importance of safety protocols in scientific investigations.
68. Color Changing Invisible Ink

The Color Changing Invisible Ink experiment offers an intriguing and fun opportunity for students to explore chemistry and learn about the concept of chemical reactions.
Learn more: Research Parent
Similar Posts:
- Top 100 Fine Motor Skills Activities for Toddlers and Preschoolers
- 37 Water Science Experiments: Fun & Easy
- Top 58 Creative Art Activities for Kids and Preschoolers
Leave a Comment Cancel reply
Save my name and email in this browser for the next time I comment.
- Science, Tech, Math ›
- Chemistry ›
A to Z Chemistry Dictionary
Look Up Definitions of Important Chemistry Terms
- Chemical Laws
- Periodic Table
- Projects & Experiments
- Scientific Method
- Biochemistry
- Physical Chemistry
- Medical Chemistry
- Chemistry In Everyday Life
- Famous Chemists
- Activities for Kids
- Abbreviations & Acronyms
- Weather & Climate
- Ph.D., Biomedical Sciences, University of Tennessee at Knoxville
- B.A., Physics and Mathematics, Hastings College
This alphabetical chemistry dictionary offers definitions and examples of important chemistry and chemical engineering terms. For each term, a brief definition is given, and each link leads to a more comprehensive discussion of the word.
From absolute alcohol, which refers to the common name for high-purity ethanol, to zinc and zwitterion, an amino acid formed when a hydrogen ion transfers from an acid group to an amine group, you're sure to learn more with these hundreds of important chemistry terms.
A- Absolute Alcohol to Azimuthal Quantum Number
absolute alcohol - common name for high purity ethanol or ethyl alcohol.
absolute error - an expression of the uncertainty or inaccuracy of a measurement.
absolute temperature - temperature measured using the Kelvin scale.
absolute uncertainty - the uncertainty of a scientific measurement, given in the same units as the measurement.
absolute zero - the lowest possible state at which matter can exist, 0 K or -273.15°C.
absorbance - measure of the amount of light absorbed by a sample.
absorption - process by which atoms, ions, or molecules enter a bulk phase.
absorption spectroscopy - technique used to determine concentration and structure of a sample based on which wavelengths of liquid are absorbed.
absorption spectrum - graph of the amount of absorption as a function of wavelength.
absorptivity - absorption cross-section of extinction coefficient, which is the absorbance of a solution per unit path length and concentration.
accuracy - the closeness of a measurement to a true or accepted value.
acid - a chemical species that accepts electrons or donates protons or hydrogen ions.
acid anhydride - a nonmetal oxide that reacts with water to form an acidic solution.
acid-base indicator - a weak acid or weak base that changes color when the concentration of hydrogen or hydroxide ions changes in an aqueous solution.
acid-base titration - a procedure to find the concentration of an acid or base by reacting a known concentration with the unknown until the equivalence point is reached.
acid dissociation constant - Ka - a quantitative measure of how strong an acid is.
acidic solution - an aqueous solution with a pH less than 7.0.
actinides - Usually, the actinides are considered to be elements 90 (thorium) through 103 (lawrencium). Otherwise, the actinides are defined according to their common properties.
actinium - the name for the element with atomic number 89 and is represented by the symbol Ac. It is a member of the actinide group.
activated complex - an intermediate state at the maximum energy point on the reaction path that occurs as reactants are being converted into products in a chemical reaction.
activation energy - Ea - the minimum amount of energy needed for a chemical reaction to occur.
active transport - the movement of molecules or ions from a region of lower concentration to higher concentration; requires energy
activity series - list of metals ranked in order of decreasing activity, used to predict which metals displace others in aqueous solutions.
actual yield - the quantity of product experimentally obtained from a chemical reaction.
acute health effect - the effect caused by initial exposure to a chemical.
acyl group - a functional group with the formula RCO- where R is bound to carbon via a single bond.
adsorption - the adhesion of a chemical species onto a surface
adulterant - a chemical that acts as a contaminant in the context of another substance's purity.
aether - a medium believed to carry light waves in the 18th and 19th centuries.
air - the mixture of gases that make up the Earth's atmosphere, consisting mainly of nitrogen, with oxygen, water vapor, argon, and carbon dioxide.
alchemy -Several definitions of alchemy exist. Originally, alchemy was an ancient tradition of sacred chemistry used to discern the spiritual and temporal nature of reality, its structure, laws, and functions.
alcohol - a substance that contains an -OH group attached to a hydrocarbon.
aliphatic amino acid - amino acid that has an aliphatic side chain.
aliphatic compound - an organic compound containing carbon and hydrogen joined into straight chains, branched chains, or non-aromatic rings.
aliphatic hydrocarbon - a hydrocarbon containing carbon and hydrogen joined into straight chains, branched chains, or non-aromatic rings.
alkali metal - any element found in group IA (first column) of the periodic table.
alkaline - an aqueous solution with a pH greater than 7.
alkalinity - a quantitative measure of a solution's ability to neutralize an acid.
alkene - a hydrocarbon containing a double carbon-carbon bond.
alkenyl group - the hydrocarbon group formed when a hydrogen atom is removed from an alkene group.
alkoxide - an organic functional group formed when a hydrogen atom is removed from the hydroxyl group of an alcohol when it is reacted with a metal.
alkoxy group - functional group containing an alkyl group bonded to oxygen.
allotrope - a form of an elemental substance.
alloy - substance made by melting together two or more elements, at least one of which must be a metal.
alpha decay - spontaneous radioactive decay which produces an alpha particle or helium nucleus.
alpha radiation - the ionizing radiation released from radioactive decay emitting an alpha particle.
aluminum or aluminium - the name for the element with atomic number 13 and is represented by the symbol Al. It is a member of the metal group.
amalgam - any alloy of mercury and one or more other metals.
americium - radioactive metal with element symbol Am and atomic number 95.
amide - functional group containing a carbonyl group linked to a nitrogen atom.
amine - compound in which one or more hydrogen atom in ammonia is replaced by an organic functional group.
amino acid - an organic acid containing a carboxyl (-COOH) and amine (-NH 2 ) functional group along with a side chain.
amorphous - term describing a solid that does not have a crystalline structure.
amphiprotic - species that can both accept and donate a proton or hydrogen ion.
amphoteric - substance capable of acting as either an acid or a base.
amphoteric oxide - oxide that can act as either an acid or a base in a reaction to produce a salt and water.
amu - atomic mass unit or 1/12th the mass of an unbound atom of carbon-12.
analytical chemistry - chemistry discipline that studies the chemical composition of materials and tools used to examine them.
angstrom - unit of length equal to 10 -10 meters.
angular momentum quantum number - ℓ, the quantum number associated with the angular momentum of an electron.
anhydrous - describes a substance that does not contain water or else is as concentrated as it can get.
anion - an ion with a negative electrical charge.
anode - electron where oxidation occurs; positive charged anode
antibonding orbital - molecular orbital with an electron outside the region between the two nuclei.
anti-Markovnikov addition - an addition reaction between an electrophilic compound HX and either an alkene or alkyne in which the hydrogen atom bonds to the carbon with the least number of hydrogen atoms and X bonds to the other carbon.
antimony - Antimony is the name for the element with atomic number 36 and is represented by the symbol Kr. It is a member of the metalloid group.
anti-periplanar - periplanar conformation where the dihedral atom between atoms is between 150° and 180°.
aqueous - describes a system containing water.
aqueous solution - a solution in which water is the solvent.
aqua regia - mixture of hydrochloric and nitric acids, capable of dissolving gold, platinum, and palladium.
argon - Argon is the name for the element with atomic number 18 and is represented by the symbol Ar. It is a member of the noble gases group.
aromatic compound - an organic molecule that contains a benzene ring.
Arrhenius acid - species that dissociates in water to form protons or hydrogen ions.
Arrhenius base - species that increases the number of hydroxide ions when added to water.
arsenic - metalloid with element symbol As and atomic number 33.
aryl - a functional group derived from a simple aromatic ring when one hydrogen is removed from the ring.
astatine - Astatine is the name for the element with atomic number 85 and is represented by the symbol At. It is a member of the halogen group.
atom - the defining unit of an element, which cannot be subdivided using chemical means.
atomic mass - average mass of atoms of an element.
atomic mass unit (amu) - 1/12th the mass of an unbound atom of carbon-12, used to represent atomic and molecular masses.
atomic number - the number of protons in the nucleus of an atom of an element.
atomic radius - value used to describe the size of an atom, usually half the distance between two atoms just touching each other.
atomic solid - solid in which atoms are bonded to other atoms of the same type.
atomic volume - volume occupied by one mole of an element at room temperature.
atomic weight - average mass of atoms of an element.
atmosphere - surrounding gases, such as the gases surrounding a planet that are held in place by gravity.
ATP - ATP is the acronym for the molecule adenosine triphosphate.
Aufbau principle - idea that electrons are added to orbitals as protons are added to an atom.
austenite - the face-centered cubic crystalline form of iron.
Avogadro's Law - relation that states equal volumes of all gases contain the same number of molecules at the same pressure and temperature.
Avogadro's number - the number of particles in one mole of a substance; 6.0221 x 10 23
azeotrope - a solution that retains its chemical composition when distilled.
azimuthal quantum number - the quantum number associated with the angular momentum of an electron, determining the shape of its orbital.
B Definitions - Background Radiation to Buffer
background radiation - radiation from external sources, typically from cosmic radiation and radioisotope decay.
back titration - titration in which the analyte concentration is determined by reacting it with a known quantity of excess reagent.
balanced equation - chemical equation in which the number and type of atoms and the electric charges are the same on both the reactant and product sides of the equation.
Balmer series - the part of the hydrogen emission spectrum for electron transitions n=2 and n>2, There are four lines in the visible spectrum.
barium - alkaline earth metal with element symbol Ba and atomic number 56.
barometer - instrument used to measure atmospheric pressure.
base - chemical species that either accepts protons or else donates electrons or hydroxide ions.
base anhydride ( basic anhydride ) - a metal oxide formed from the reaction between water and a basic solution.
base metal - any metal besides a precious or noble metal used for jewelry or in industry.
basic - alkaline or having a pH > 7.
basic solution - aqueous solution containing more hydroxide ions than hydrogen ions; solution with pH > 7.
Beer's law (Beer-Lambert Law) - law that states the concentration of a solution is directly proportional to its light absorbance.
berkelium - radioactive metal with element symbol Bk and atomic number 97.
beryllium - alkaline earth metal with element symbol Be and atomic number 4.
beta decay - type of radioactive decay that results in spontaneous emission of a beta particle.
beta particle - an electron or positron emitted during beta decay.
beta radiation - ionizing radiation from beta decay in the form of an energetic electron or positron.
binary acid - an acidic binary compound in which one element is hydrogen and the other element is another nonmetal.
binary compound - a compound made up of two elements (e.g., HF).
binding energy - energy needed to remove an electron from an atom or to separate a proton or neutron from the atomic nucleus.
biochemistry - Biochemistry is the chemistry of living things.
bismuth - Bismuth is the name for the element with atomic number 83 and is represented by the symbol Bi. It is a member of the metal group.
bitumen - natural mixture of polycyclic aromatic hydrocarbons (PAHs).
black light - a lamp that emits ultraviolet radiation or the invisible radiation emitted by it.
block copolymer - copolymer formed by repeating monomer subunits.
bohrium - transition metal with element symbol Bh and atomic number 107.
boiling - phase transition from the liquid to gas state.
boiling point - temperature at which a liquid's vapor pressure is equal to the external gas pressure.
boiling point elevation - the increase in a liquid boiling point caused by adding another compound to it.
bond - a chemical link formed between atoms in molecules and molecules and ions in crystals.
bond angle - the angle formed between two adjacent chemical bonds within the same atom.
bond-dissociation energy - energy required to homolytically break a chemical bond.
bond energy - quantity of energy needed to break one mole of molecules into component atoms.
bond enthalpy - enthalpy change resulting when one mole of bonds in a species are broken at 298 K.
bond length - the equilibrium distance between atomic nuclei or groups of nuclei that share a chemical bond.
bond order - a measure of the number of electrons involved in chemical bonds between two atoms in a molecule; usually equal to the number of bonds between the atoms.
boron - Boron is the name for the element with atomic number 5 and is represented by the symbol B. It is a member of the semimetal group.
Boyle's law - ideal gas law that states the volume of a gas is inversely proportional to its absolute pressure, assuming constant temperature.
branched chain alkane - an alkane with alkyl groups bonded to the central carbon chain. The molecules are branched, but all C-C bonds are single bonds.
brass - Brass is defined as an alloy of copper and zinc .
bromine - Bromine is the name for the element with atomic number 35 and is represented by the symbol Br. It is a member of the halogen group.
Bronsted-Lowry acid - species that yields hydrogen ions.
Bronsted-Lowry base - species that accepts hydrogen ions in a reaction.
bronze - Bronze is an alloy of copper, usually containing tin as its main addition.
buffer - either a weak acid and its salt or a weak base and its salt that forms an aqueous solution that resists pH changes.
C - Cadmium to Current
cadmium - Cadmium is the name for the element with atomic number 48 and is represented by the symbol Cd. It is a member of the transition metals group.
caffeine - Caffeine is a chemical substance naturally found in tea and coffee and added to colas.
calcium - Calcium is the name for the element with atomic number 20 and is represented by the symbol Ca. It is a member of the alkaline earth metal group.
calorie - unit of thermal energy; the amount of energy required to raise the temperature of 1 gram of water 1 degree C or K at standard pressure.
calorimeter - instrument designed to measure the heat flow of a chemical reaction or physical change.
capillary action - the spontaneous flow of liquid into a narrow tube or porous material.
carbon - Carbon is the name for the element with atomic number 6 and is represented by the symbol C. It is a member of the nonmetal group.
carbonate - an ion consisting of one carbon bonded to three oxygen atoms (CO 3 2- ) or a compound containing this ion.
carbonyl - functional group consisting of a carbon atom double bonded to oxygen, C=O.
carboxyl group - functional group consisting of a carbon double bonded to oxygen and single bonded to a hydroxyl (-COOH).
catalyst - substance that increases the chemical reaction rate by decreasing its activation energy.
catenation - binding of an element to itself via covalent bonds, forming a chain or ring
cathode - electrode where reduction occurs; usually the negative electrode.
cathode ray tube - a vacuum tube with a source of electrons, a fluorescent screen, and means of accelerating and deflecting the electron beam.
cation - ion with a positive electrical charge.
Celsius temperature scale - temperature scale where 0°C and 100°C are defined as the freezing and boiling points of water, respectively.
cerium - rare earth metal with element symbol Ce and atomic number 58.
cesium - Cesium is the name for the element with atomic number 55 and is represented by the symbol Cs. It is a member of the alkali metal group.
cetane number (CN) - value that describes the combustion quality of diesel fuel, based on the delay between injection and ignition.
chain reaction - set of chemical reactions in which products become reactants of another reaction.
charge - an electrical charge, a conserved property of subatomic particles determining their electromagnetic interaction.
Charles's law - ideal gas law that states the volume of an ideal gas is directly proportional to absolute temperature, assuming constant pressure.
chelate - organic compound formed by bonding a polydentate ligand to a central metal atom, or the act of forming such a compound.
chemical - any matter or substance that has mass.
chemical change - process by which one or more substances are altered to form new substances.
chemical energy - energy contained in the internal structure of an atom or molecule.
chemical equation - description of a chemical reaction, including the reactants, products, and direction of the reaction.
chemical equilibrium - state of a chemical reaction where the concentration of the reactants and products remains stable over time.
chemical formula - expression which states the number and type of atoms in a molecule.
chemical kinetics - the study of chemical processes and rates of reactions.
chemical property - characteristic which may be observed when matter undergoes a chemical change.
chemical reaction - a chemical change in which reactants form one or more new products.
chemical symbol - one- or two-letter representation of a chemical element (e.g., H, Al).
chemiluminescence - light emitted as a result of a chemical reaction
chemistry - study of matter and energy and the interactions between them
Cherenkov radiation - Cherenkov radiation is the electromagnetic radiation emitted when a charged particle moves through a dielectric medium faster than the velocity of light in the medium.
chiral center - the atom in a molecule bonded to four chemical species, allowing optical isomerism.
chirality - Chirality or chiral describes a non-superimposable mirror image, like left and right hands. Usually in chemistry, the term is used to describe a pair of molecules that have the same formulas but form a pair of structures.
chlorine - halogen with atomic number 17 and element symbol Cl.
chlorofluorocarbon - A chlorofluorocarbon or CFC is a compound that contains atoms of chlorine, fluorine, and carbon.
chromatography - group of techniques used to separate mixture components by passing the mixture through a stationary phase.
chromium - Chromium is the name for the element with atomic number 24 and is represented by the symbol Cr. It is a member of the transition metals group.
closed system - thermodynamic system in which mass is conserved within the system, but energy can freely enter or exit.
coagulation - the gelling or clumping of particles, usually in a colloid.
cobalt - transition metal that is atomic number 27 with element symbol Co.
coenzyme - substance that works with an enzyme to aid its function or initiate its action.
cohesion - measure of how well molecules stick to each other or group together.
collagen - an important family of proteins found in humans and other animals, found in skin, cartilage, blood vessels, and tendons.
colligative properties - properties of a solution that depend on the number of particles in a volume of solvent.
colloid - a homogenous mixture in which dispersed particles do not settle out.
combined gas law - law which states the ratio of the product of pressure and volume, divided by the absolute temperature, is a constant value.
combination reaction - reaction in which two reactants combine to form a single product.
combustion - chemical reaction between a fuel and oxidizer that yields energy (usually heat and light).
common-ion effect - suppressing effect an electrolyte has on the ionization of another electrolyte that shares a common ion.
compound - chemical species formed when two or more atoms form a chemical bond.
complex ion - ion in which a central metal ion is bonded to one or more ions or molecules.
concentrated - having a large ratio of solute to solvent.
concentration - an expression of the quantity of a substance in a defined volume.
condensation - state of matter changes from vapor phase to liquid phase.
condensation reaction - chemical reaction in which one of the products is water or ammonia, also known as a dehydration reaction.
condensed formula - chemical formula in which atom symbols are listed in the order they appear in the molecular structure, with limited bond dashes.
conductor - material that permits the flow of energy (e.g., electrical conductor, thermal conductor).
conformer - an isomer that differs from another isomer by rotation around a single bond.
congener - member of the same group of elements of the periodic table (e.g., iodine and chlorine).
conjugate - multiple chemistry definitions, referring to Bronsted acids and bases, a compound formed by combining other compounds, or the overlap of p-orbitals across a sigma bond.
conjugate acid - HX, a compound differing from a base X by a proton.
conjugate base - the species that gains a proton in an acid-base reaction.
conservation of energy - law which states energy can change forms but may not be created or destroyed.
conservation of mass - law that states, in a closed system, matter can change forms but not be created or destroyed.
controlled variable - variable that a scientist holds constant in an experiment; the control or constant variable
conversion factor - numerical ratio that converts a measurement from one unit into another.
coordinate bond - covalent bond between two atoms in which one atom supplies both electrons for the bond.
coordination compound - compound containing one or more coordinate bonds.
coordination number - number of atoms bonded to a central atom.
copernicium - radioactive element with symbol Cn and atomic number 112.
copper - Copper is the name for the element with atomic number 29 and is represented by the symbol Cu. It is a member of the transition metals group.
corrosion - irreversible damage to a material or tissue due to a chemical reaction.
corrosive - having the power to cause irreversible chemical damage upon contact.
Coulomb's law - law which states the force between two charges is proportional to the quantity of both charges and inversely proportional to the square of the distance between them.
covalent bond - chemical link between atoms or ions in which the electron pairs are more or less evenly shared between them.
covalent compound - molecule that contains covalent chemical bonds.
covalent radius - half the diameter of the part of an atom that participates in a covalent bond.
crenation - forming a scalloped shape upon exposure to a hypertonic solution.
critical point - critical state; point at which two phases of matter become indistinguishable from one another.
cyrogenics - study of matter at extremely low temperatures
crystal - matter in which atoms, ions, or molecules are packed into an ordered, repeating three-dimensional pattern.
crystal field splitting - the difference in energy between the d orbitals of ligands.
crystallize - solidification of matter into the highly ordered form of a crystal.
curium - radioactive metal with element symbol Cm and atomic number 96.
current - rate of flow of electricity.
D - Dalton's Law to Dysprosium
Dalton's Law - relation stating the total pressure of a gaseous mixture equals the sum of the partial pressure of the component gases.
darmstadtium - Darmstadtium is the name for the element with atomic number 110 and is represented by the symbol Ds. Darmstadtium was formerly known as ununnilium with symbol Uun. It is a member of the transition metals group.
dative bond - covalent bond between atoms in which one atom provides both electrons for the bond.
daughter isotope - product formed after a radioisotope (the parent) undergoes radioactive decay.
de Broglie Equation - equation describing the wave properties of matter, stated as wavelength equals Planck's constant divided by the product of mass and velocity.
decantation - method of separating mixtures by removing the liquid layer from a precipitate.
decomposition reaction - chemical reaction in which a single reactant yields two or more products.
deflagration - type of combustion in which flame propagation is less than 100 m/s and overpressure is less than 0.5 bar.
dehydration reaction - chemical reaction between two compounds in which one of the products is water.
deliquescence - process by which a soluble substance picks up water vapor from the atmosphere to form a solution.
delocalized electron - any electron in an ion, atom, or molecule that is no longer associated with a particular atom or single covalent bond.
density - mass per unit volume.
dependent variable - variable being measured (tested) in response to changing the independent variable.
deposition - settling of sediment or particles onto a surface or the phase change from the vapor to solid phase.
deprotonation - chemical reaction in which a radical removes a proton from a molecule.
derived unit - an SI unit made from a combination of the base units (e.g., Newton is kg·m/s 2 ).
desiccant - chemical agent that picks up water, often used for drying.
desublimation - phase change from vapor to solid.
detergent - cleaning agent with general structure R-SO 4 - , Na + , where R is a long-chain alkyl group.
diamagnetic - not attracted to a magnetic field, generally because the material does not contain unpaired electrons.
diffusion - movement of a fluid from a region of higher concentration to a lower concentration.
dilute - solution containing a small amount of solute relative to the amount of solvent.
dipole - a separation of electrical or magnetic charges.
dipole moment - measure of the separation of two opposite electrical charges.
diprotic acid - acid that can donate two hydrogen atoms or protons per molecule in an aqueous solution.
direct proportion - relation between two variables such that their ratio is a constant value.
disaccharide - carbohydrate formed when two monosaccharides bond, removing a molecule of water from their structure.
displacement reaction - chemical reaction in which the cation or anion of one reactant is replaced by one from another reactant.
disproportionation - chemical reaction (usually redox) where a molecule forms two or more dissimilar products.
dissociation reaction - chemical reaction in which a reactant breaks into two or more parts.
dissolve - a solute passing into solution, usually a solid going to the liquid phase.
distillate - vapor formed by a distillation, which may be condensed into a liquid for collection.
distillation - technique of heating a liquid to form a vapor, which is cooled to separate components of the liquid based on volatility or boilings.
divalent cation - positive charged ion with a valence of 2.
DNA - deoxyribonucleic acd, an organic molecule that codes for proteins.
double bond - chemical bond in which two electron pairs are shared between two atoms.
double replacement reaction - chemical reaction in which two reactants exchange anions/cations to form two new products using the same ions.
dry ice - the solid form of carbon dioxide
dubnium - transition metal with element symbol Db and atomic number 105.
ductile - able to be stretched into a wire without breaking.
dynamic equilibrium - chemical equilibrium between the forward and reverse reaction in which the rates of reaction are equal to each other.
dysprosium - rare earth metal with element symbol Dy and atomic number 66.
E - Effective Nuclear Charge to Extensive Property
effective nuclear charge - net charge an electron experiences in an atom that has multiple electrons.
effervescence - foaming or bubbling when gas is evolved by a liquid or solid.
efflorescence - process by which a hydrate loses water of hydration.
effusion - movement of gas through a pore or capillary into a vacuum or another gas.
einsteinium - Einsteinium is the name for the element with atomic number 99 and is represented by the symbol Es. It is a member of the actinide group.
elasticity - physical property of matter describing the ability to return to original shape after deformation.
electrical conductivity - measure of a substance's ability to carry an electrical current.
electrical resistivity - measure of how much a material resists carrying an electrical current.
electrochemical cell - device that generates a potential difference between electrodes via chemical reactions.
electrochemistry - scientific study of reactions and species formed at the interface between an electrolyte and a conductor, where electron transfer occurs.
electromotive force - emf - the electric potential generated by either an en electrochemical cell or changing magnetic field.
electrode - the anode or cathode of an electrical cell.
electrolysis - passage of direct current through an ion-conducting solution, producing a chemical change at the electrodes.
electrolyte - a substance that forms ions in aqueous solution.
electrolytic cell - type of electrochemical cell in which the flow of electric energy from an external source enables a redox reaction.
electromagnetic radiation - light; self-propagating energy that has electric and magnetic field components.
electron - stable negatively charged subatomic particle.
electron affinity - measure of the ability of an atom to accept an electron.
electron capture (EC) - form of radioactive decay in which the atomic nucleus absorbs a K or L shell electron, converting a proton into a neutron.
electron cloud - region of negative charge surrounding the atomic nucleus that has a high probability of containing electrons.
electron configuration - description of the population of the electronic energy sublevels of an atom.
electron density - representation of the probability of finding an electron in a specific region around an atom or molecule.
electron domain - the number of lone electron pairs or bond locations around an atom or molecule.
electronegativity - property of an atom that reflects its ability to attract electrons in a chemical bond.
electron pair repulsion - principle that electron pairs surrounding a central atom orient themselves as far apart as possible; used to predict geometry.
electron-sea model - model of metallic bonding in which cations are described as fixed points in a mobile sea of electrons.
electron spin - property of an electron related to its spin about an axis, described by a quantum number as either +1/2 or -1/2.
electrophile - atom or molecule that accepts an electron pair to form a covalent bond.
electroplating - process of adding a metal coat to a material by using a reduction reaction.
electrostatic forces - forces between particles due to their electrostatic charges.
electrum - a natural alloy of gold and silver.
element - a substance that cannot be subdivided using chemical means; identified by the number of protons in its atoms.
elementary reaction - chemical reaction in which reactants form products in a single step without a transition state.
element symbol - the one- or two-letter abbreviation of a chemical element (e.g., H, Cl).
emissions - products of a combustion reaction, aside from heat and light (e.g., carbon dioxide).
emission spectrum - range of wavelengths emitted by an atom stimulated by electricity or heat.
empirical formula - formula that shows the ratio of elements in a compound, but not necessarily their actual numbers in a molecule.
emulsifier - stabilizing agent that prevents immiscible liquids from separating.
emulsion - colloid formed from two or more immiscible liquids where one liquid contains a dispersion of the other liquid(s).
enantiomer - a member of a pair of optical isomers.
endothermic - process which absorbs thermal energy from its environment.
enediol - an alkene enol with a hydroxyl group attached to both carbon atoms of the C=C bond.
energy - the ability to do work (e.g., kinetic energy, light).
enthalpy - thermodynamic property of a system that is the sum of the internal energy and the product of pressure and volume.
enthalpy change - the energy change of a system at constant pressure.
enthalpy of atomization - quantity of enthalpy change when chemical bonds are broken in a compound to form individual atoms.
enthalpy of reaction - difference between total enthalpy of products and total enthalpy of reactants of a chemical reaction.
entropy - measure of the disorder of a system.
enzyme - An enzyme is a protein that functions as a catalyst for a chemical reaction.
equilibrium constant - ratio of the equilibrium concentration of products raised to the power of their stoichiometric coefficients to the equilibrium concentration of the reactants raised to the power of their stoichiometric coefficients.
equivalence point - point in a titration where the titrant completely neutralizes the analyte.
erbium - Erbium is element atomic number 68 on the periodic table.
essential amino acid - amino acid needed in the diet because an organism cannot synthesize it.
ester - RCO 2 R′, where R is the hydrocarbon parts of the carboxylic acid and R′ is the alcohol.
ether - organic compound containing two aryl or alkyl groups bound to an oxygen, R-O-R'.
europium - Europium is the name for the element with atomic number 63 and is represented by the symbol Eu. It is a member of the lanthanide group.
eutectic - homogeneous solid mixture of at least two types of atoms or molecules that form a superlattice (usually a mix of alloys).
evaporation - process characterized by a spontaneous transition of molecules from the liquid phase to vapor phase.
excess reactant - reactant left over in a reaction because it is present in a great amount than needed to react with the limiting reactant.
excited state - atom, ion, molecule, or subatomic particle in a higher energy level than its ground state.
exergonic - releasing energy to its surroundings.
exothermic - releasing energy to the environment in the form of heat; a type of exergonic process
exothermic reaction - a chemical reaction that releases heat.
extensive property - property of matter that depends on the quantity of matter that is present (e.g., volume).
F - F Orbital to Fusion
f orbital - electron orbital with l = 3 for the angular momentum quantum number,
family - a group of elements that share similar properties.
Fa raday constant - a physical constant equal to the electric charge of one mole of electrons, 96485.33 C/mol.
fat - triesters of glycerol and fatty acids that are soluble in organic solvents, but generally insoluble in water.
fatty acid - a carboxylic acid with a long hydrocarbon side chain.
feedstock - any unprocessed material used as a supply for a manufacturing process.
fermium - Fermium is the name for the element with atomic number 100 and is represented by the symbol Fm. It is a member of the actinide group .
first law of thermodynamics - law which states the total energy of a system and its surroundings is a constant value; the law of conservation of energy.
fire point - the lowest temperature a vapor will initiate and sustain combustion.
fission - the splitting of an atomic nucleus, which results in two or more lighter nuclei and a release of energy.
flame test - an analytical technique used to identify ions based on their emission spectrum in a flame.
flammable - easily ignited or capable of sustained combustion.
fluid - a substance that flows under applied shear stress, including liquids, gases, and plasma.
fluorescence - luminescence released when an atom absorbs electromagnetic radiation and emits a photon when the electron falls to a lower energy state.
foam - a substance containing gas bubbles trapped within a liquid or solid.
force - a push or pull on a mass, with both magnitude and direction (vector).
formal charge - the difference between the number of valence electrons of an atom and the number of electrons associated with the atom (e.g., in a chemical bond).
formation reaction - reaction in which one mole of a product is formed.
formula mass or formula weight - the sum of the atomic weights of the atoms in a compound's empirical formula.
fractional distillation - process which separates components of a mixture according to their boiling points.
francium - alkali metal with element symbol Fr and atomic number 87.
free energy - the amount of internal energy of a system that is available to do work.
free radical - an atom or molecule with an unpaired electron.
freezing - process in which a liquid changes to a solid.
freezing point - temperature at which a liquid transitions to a solid (not always the same as melting point).
freezing point depression - lowering the freezing point of a liquid by adding another compound to it.
frequency - number of times a point on a wave passes a reference point in one second.
functional groups or functional moiety - group of atoms in a molecule that are responsible for characteristic reactions and properties.
fusion - combining light atomic nuclei to form a heavier nucleus, accompanied by the release of energy.
G - Gadolinium to Group
gadolinium - rare earth metal with element symbol Gd and atomic number 64.
gallium - metal with element symbol Ga and atomic number 31. galvanic cell - electrochemical cell where reactions between dissimilar conductors occur through a salt bridge and electrolyte.
gamma radiation - high energy ionizing photons, originating from the atomic nucleus.
gas - state of matter characterized by having neither a defined shape nor defined volume.
gas constant (R) - the constant in the Ideal Gas Law; R = 8.3145 J/mol·K.
Gay-Lussac's law - form of the ideal gas law that states the pressure of an ideal gas is directly proportional to its absolute (Kelvin) temperature when volume is held constant.
gel - a type of sol where the solid particles are held in a mesh to form a rigid or semi-rigid mixture.
geometric isomer - molecules with the same number and type of atoms as each other, but with different geometrical configurations. Also called cis-trans or configurational isomerism.
germanium - metalloid with element symbol Ge and atomic number 32.
Gibbs free energy - a measure of the potential for reversible or maximum work done by a system at constant pressure and temperature.
glass - an amorphous solid.
glycosidic bond - a covalent bond between a carbohydrate and a functional group or another molecule.
gold - yellow-colored transition metal with element symbol Au and atomic number 79.
Graham's Law - relation stating the rate of effusion of a gas is inversely proportional to the square root of its molecular mass or density.
grain alcohol - purified form of ethyl alcohol made from distilling fermented grain.
gram - unit of mass equal to the mass of one cubic centimeter of water at 4°C.
gram molecular mass - the mass in grams of one mole of a molecular substance.
gravimetric analysis - a set of quantitative analytical techniques based on measurement of a sample's mass.
green chemistry - branch of chemistry concerned with lessening the environmental effect of chemicals, including development of new materials and processes.
ground state - the lowest energy state of an atom, ion, molecule, or subatomic particle.
group - a vertical column on the periodic table consisting of elements that share periodic properties.
H - Haber Process to Hypothesis
Haber process - method of making ammonia or fixing nitrogen by reacting nitrogen and hydrogen gas
hafnium - transition metal with element symbol Hf and atomic number 72.
half-cell - half of an electrolytic or voltaic cell, serving as the site of either oxidation or reduction.
half-life (t 1/2 ) - time required to convert half of the reactant to a product or the time required for half of a radioactive isotope to decay into its daughter isotope.
halide ion - a singlet halogen atom, which has a charge of -1 (e.g., Cl - )
halogen - an element in Group VIIA of the periodic table (e.g., Br, Cl).
halogenated hydrocarbon - a hydrocarbon that contains one or more halogen atoms.
hard water - water that contains high amounts of calcium and/or magnesium cations.
hassium - transition metal that is atomic number 108 with element symbol Hs.
heat - energy that flows between matter samples because of a temperature difference.
heat capacity - quantity of heat needed to raise the temperature of a sample by a specified amount.
heat of formation ( ΔH f ) - amount of heat absorbed or released during formation of a pure substance from its elements at constant pressure.
heat of fusion ( ΔH fus ) - the change in enthalpy (heat) for the conversion of one gram or mole of a solid to a liquid at constant temperature and pressure.
heavy metal - a dense metal that is toxic at low concentrations.
Heisenberg uncertainty principle - principle that states it is impossible to determine both the position and momentum of a particle at once with perfect accuracy.
helium - Helium is the name for the element with atomic number 2 and is represented by the symbol He. It is a member of the noble gases group.
Henderson-Hasselbalch equation - an approximation that relates the pH or pOH of a solution, the pK a or pK b , and the ratio of concentration of dissociated species.
Henry's Law - law that states the mass of a gas that will dissolve into solution is directly proportional to the partial pressure of the gas above the solution.
Hess's Law - law that states the energy change in an overall reaction equals the sum of the energy changes in its individual (partial) reactions.
heterogeneous -- consisting of dissimilar components.
heterogeneous mixture - a mixture that lacks a uniform composition such that at least two components are present with identifiable properties.
heterogeneous reaction - chemical reaction in which reactants are different phases from each other.
holmium - rare earth metal with element symbol Ho and atomic number 67.
homogeneous - uniform through its volume.
homopolymer - polymer in which every mer unit is identical.
hybrid orbital - orbital formed by the combination of two or more atomic orbitals.
hydration reaction - reaction in which a hydrogen and hydroxyl ion are attached to a carbon in a C-C double bond.
hydrocarbon - molecule consisting entirely of carbon and hydrogen atoms.
hydrogen - element with atomic number 1 and symbol H.
hydrogen bond - attractive interaction between a hydrogen bonded to an electronegative atom and a different electronegative atom.
hydrogenation - reduction reaction that produces hydrogen (usually as H 2 ).
hydrolysis - decomposition reaction in which one reactant is water. Reverse of a condensation reaction.
hydrometer - instrument used to measure the relative densities of two liquids.
hydronium ion - the H 3 O + cation.
hydrophobic - property of repelling water.
hydroxyl group - functional group consisting of a hydrogen atom covalently bonded to an oxygen atom (-OH).
hygroscopic - able to absorb or adsorb water from the surroundings.
hypertonic - having higher osmotic pressure than another solution.
hypothesis - prediction of an event or proposed explanation of a phenomenon.
I - Ideal Gas to IUPAC
ideal gas - gas in which molecules have negligible size and kinetic energy dependent only on temperature.
ideal gas constant - physical constant in the Ideal Gas Law, equal to the Boltzmann constant but with different units.
ideal gas law - PV = nRT where P is pressure, V is volume, n is number of moles, R is the ideal gas constant, and T is temperature.
immiscible - property of two substances being unable to combine to form a homogeneous mixture; unable to mix
independent variable - the variable that is controlled or changed in an experiment to test its effect on the dependent variable.
indicator - substance that undergoes a visible change when its conditions change (e.g., a pH indicator).
indium - metal with element symbol In and atomic number 49.
inductive effect - effect a chemical bond has on the orientation of adjacent bonds in a molecule.
inhibitor - substance that slows or prevents a chemical reaction.
inorganic chemistry - study of chemistry of molecules of non-biological origin (not containing C-H bonds).
insoluble - unable to dissolve in a solvent.
intensive property - property of matter that is independent of the quantity of matter in a sample.
intermolecular force - the sum of all forces between neighboring molecules.
internal energy - the total energy (U) of a closed system.
intrinsic property - property of matter that is independent of the quantity of matter present.
intermediate - substance formed in a middle step between reactants and final products.
inverse proportion - relationship between variable such that their product is a constant value.
iodine - Iodine is the name for the element with atomic number 53 and is represented by the symbol I. It is a member of the halogen group.
ion - atom or molecule that has a different number of protons than electrons and thus a net electrical charge.
ionic - pertaining to carrying a net electrical charge at the atomic or molecular level.
ionic bond - chemical link between atoms caused by electrostatic force between oppositely charged ions.
ionic compound - compound formed by ions bonding together due to electrostatic forces (differing electronegativity values).
ionic equation - chemical equation in which electrolytes in aqueous solution are written as dissociated ions.
ionic radius - half the distance between two ions just touching each other.
ionization energy - energy required to completely remove an electron from a gaseous atom of ion.
iridium - Iridium is the name for the element with atomic number 77 and is represented by the symbol Ir. It is a member of the transition metals group.
iron - Iron is the name for the element with atomic number 26 and is represented by the symbol Fe. It is a member of the transition metals group.
isoelectronic - chemical species that have the same electronic structure and thus same number of valence electrons.
isolated system - thermodynamic system that can't exchange energy or matter outside of the system.
isomer - chemical species with the same number and type of atoms as another species, but a different arrangement and thus different properties.
isomerization process - protocol in which straight chain hydrocarbons are converted into branched chain hydrocarbons.
isotopes - atoms that have the same number of protons, but different numbers of neutrons and thus different atomic weight values.
IUPAC - International Union of Pure and Applied Chemistry, an authority on chemical standards.
Chemistry Definitions Starting With the Letter J
joule - SI unit of energy equal to the kinetic energy of a 1 kilogram mass moving at 1 meter per second.
K - Kelvin Temperature to Krypton
Kelvin temperature scale - an absolute temperature scale with 100 degrees between the freezing and boiling points of water (although values are given without degrees by convention).
keratin - a fibrous protein produced by chordates. It may be found in hair, skin, claws, and wool.
ketone - compound containing a carbonyl functional group (C=O) between two groups of atoms
kilo - prefix meaning "one thousand".
kilopascal (kPa) - unit of pressure exerted by a 10-gram mass on a square centimeter. There are 1000 Pa in 1 kPa.
kinetic energy - energy associated with motion.
krypton - element 36 on the periodic table with symbol Kr.
L - Labile Complex to Lutetium
labile complex - a complex ion that quickly reaches equilibrium with ligands in the surrounding solution.
lanthanides - subset of transition metals characterized by filling of the 4f sublevel, usually atomic number 58-71.
lanthanum - element atomic number 57 with element symbol La.
lattice energy - enthalpy change of the process by which opposite-charged ions in a gas combine to form a solid ionic lattice.
law - a general rule that explains a body of scientific observations. Laws are stated in words, but expressed by mathematical equations.
Law of Chemical Equilibrium - an expression of the relationship between the concentration of reactants and products of a chemical reaction mixture at equilibrium.
Law of Combining Volumes - relation that states the volumes of gases in a chemical reaction are present in the ratio of small integers under conditions where all gases are at the same temperature and pressure.
Law of Conservation of Energy - law that states energy can neither be created nor destroyed, although it may change from one form into another.
Law of Conservation of Mass - law that states matter in a closed system may be neither created nor destroyed, although it may change forms.
Law of Constant Composition - chemistry law that states samples of a pure compound contain the same elements in the same proportions by mass.
Law of Definite Proportions - law that states all samples of a compound contain the same proportion of elements by mass.
Law of Multiple Proportions - law that states elements combine in ratios of small whole numbers to form molecules.
lawrencium - actinide with element symbol Lr and atomic number 103.
lead - metal with element symbol Pb and atomic number 82.
Le Chatelier's Principle - principle that says that equilibrium of a chemical system will shift in the direction to relieve stress.
Lewis acid - chemical species that can act as an electron pair acceptor.
Lewis base - a substance that is an electron pair donor.
Lewis acid base reaction - chemical reaction that forms at least one covalent bond between an electron pair donor (Lewis base) and electron pair acceptor (Lewis acid).
Lewis structure - representation of a molecule that uses dots to show electrons around atoms and lines to show covalent bonds.
ligand - a chemical species that donates or shares at least one electron via a covalent bond with a central ion or atom.
limiting reactant - the reactant that determines how much product may result from a chemical reaction.
lipid - class of fat-soluble molecules, also known as oils and fats
liquefaction - process of converting material from a solid or gas phase into the liquid phase.
liquid - state of matter characterized by having a definite volume but not a definite shape.
lithium - alkali metal with atomic number 3 and element symbol Li.
litmus paper - filter paper used as a pH paper that has been treated with a water-soluble dye obtained from lichens.
London dispersion force - weak intermolecular force between atoms or molecules in close proximity to each other, due to electron repulsion.
lone pair - an electron pair in the outer shell of an atom that is not shared or bonded with another atom.
lutetium - rare earth metal with element symbol Lu and atomic number 71.
M - Macromolecule to Muriatic Acid
macromolecule - molecule containing a very large number of atoms, usually more than 100.
Madelung's rule - rule that describes filling of electron orbitals in atoms due to shielding of nuclear charge by inner electrons.
magnesium - Magnesium is the name for the element with atomic number 12 and is represented by the symbol Mg. Magnesium is an alkaline earth metal.
main group elements - any of the elements in the s and p blocks of the periodic table.
malleable - able to be shaped or pounded with a hammer, usually applied to metals.
manganese - element with atomic number 25 and element symbol Mn.
manometer - device used to measure gas pressure.
mass - amount of matter a substance contains or property of matter that resists acceleration.
mass defect - difference between the mass of an atom and the sum of the masses of its protons, neutrons, and electrons.
mass number - whole number integer that is the sum of the number of protons and neutrons in the atomic nucleus.
mass percentage - concentration calculated as mass of a component divided by total mass of mixture or solution; w/w%.
mass spectroscopy - analytical technique used to separate and/or identify components of a mixture based on mass and electrical charge.
matter - anything that has mass and occupies volume.
measurement - quantitative or numerical data describing an object or event.
medicinal chemistry - branch of chemistry concerned with design, synthesis, and study of pharmaceuticals.
meitnerium - radioactive transition metal with element symbol Mt and atomic number 109.
melting - phase change of matter from solid to liquid.
melting point - temperature at which the solid and liquid phase of matter coexist in equilibrium.
mendelevium - actinide with atomic number 101 and element symbol Md.
meniscus - phase boundary between a liquid in a container and a gas, curved due to surface tension.
mercaptan - organic sulfur compound containing an alkyl or aryl group and a thiol group.
mercapto group - functional group consisting of a sulfur bonded to a hydrogen, with a formula of -SH.
mercury - transition metal with element symbol Hg and atomic number Hg.
metabolism - set of biochemical reactions that store chemical energy and convert it into a form an organism can use.
metal - substance that has high conductivity and other metallic properties, including tendency to form cations, often identified by group on the periodic table.
metallic character - set of chemical properties associated with metals, including the ability to lose outer valence electrons to form cations.
metallic compound - chemical compound that contains one or more metal atoms.
metalloid - element with properties intermediate between those of metals and nonmetals (e.g., silicon).
meter - either (a) the base unit of length in the SI system or (b) a device used to measure a quantity.
methy l - functional group containing a carbon bonded to three hydrogen atoms, -CH 3 .
microliter - unit of volume that is one-millionth of a liter one cubic millimeter.
micron - unit of length equal to one-millionth of a meter; a micrometer.
mineral acid - any inorganic acid (e.g., sulfuric acid).
miscible - soluble or able to be mixed to form a solution, typically applied to fluids.
mixture - combination of two or more substances such that each retains its separate chemical identity (e.g., salt and flour).
moderator - material that slows or moderates the speed of neutrons.
Mohs scale - Mohs scale is a relative scale rating the hardness of a mineral. A mineral with a high Mohs number is able to mark a mineral with a lower Mohs number.
moiety - group of atoms in a molecule that are responsible for its characteristic chemical behavior.
molality - unit of concentration that is the moles of solute divided by the kilograms of solvent.
molar - refers to molarity (moles per liter of solution); e.g. a 6 M HCl solution has 6 moles of hydrochloric acid per liter of solution.
molar enthalpy of fusion - energy needed to change one mole of a substance from solid to liquid phase at constant pressure and temperature.
molar enthalpy of vaporization - energy needed to change one mole of liquid to the gas phase at constant pressure and temperature.
molarity - unit of concentration that is the number of moles of solute divided by the number of liters of solution.
molar mass - mass of one mole of a substance.
molar heat capacity - heat energy required to raise the temperature of 1 mole of a substance 1 Kelvin.
molar volume - volume of one mole of a substance.
mole - chemical mass unit equal to 6.022 x 10 23 molecules, atoms, or other particles.
molecular equation - balanced chemical equation in which ionic compounds are expressed as molecules rather than ions.
molecular formula - expression of the number and type of atoms in a molecule.
molecular geometry - description of the shape of a molecule and the relative positions of its atoms.
molecular mass - sum of the atomic masses of atoms in a molecule.
molecular orbital - wave function of an electron in a molecule.
molecular weight - sum of the atomic weights of atoms in a molecule.
molecule - chemical species formed by two or more atoms that share chemical bonds such that they form one unit.
mole fraction - unit of concentration that is the number of moles of a component divided by the total number of moles of a solution.
mole ratio - ratio or fraction comparing the numbers of moles of any two components involved in a chemical reaction.
molybdenum - transition metal with element symbol Mo and atomic number 42.
monatomic ion - an ion formed by a single atom.
monomer - a molecule that is a subunit or building block of a polymer.
monoprotic acid - acid that donates a single proton or hydrogen atom per molecule in aqueous solution.
mother liquor - solution remaining after crystals are removed from a crystallization solution.
MSDS - acronym for Material Safety Data Sheet, a written document outlining safety information about a chemical.
multiple bond - a bond formed when two or more pairs of electrons are shared between two atoms.
muriatic acid - common name for hydrochloric acid, HCl.
N - Napthenes to Nutraceutical
naphthenes - cyclic aliphatic hydrocarbons from petroleum with the general formula C n H 2n .
natural abundance - average percentage of a given isotope naturally occurring on Earth.
neodymium - rare earth metal with element symbol Nd and atomic number 60.
neon - noble gas with element symbol Ne and atomic number 10.
neptunium - actinide with element symbol Np and atomic number 94.
net ionic equation - chemical equation that lists only the species participating in the reaction.
network solid - material consisting of an array of repeating covalently bonded atoms.
neutral solution - aqueous solution with a pH of 7.
neutralization - chemical reaction between an acid and base that results in a neutral solution.
neutron - particle in the atomic nucleus that has a mass of 1 and charge of 0.
newton (N) - SI unit of force equal to the amount of force needed to accelerate a 1 kg mass 1 m/sec 2 .
nickel - Nickel is the name for the element with atomic number 28 and is represented by the symbol Ni. Nickel is a member of the transition metals group.
niobium - Niobium is the name for the element with atomic number 41 and is represented by the symbol Nb. Niobium is also called Columbium and is a transition metal.
nitrogen - Nitrogen is the name for the element with atomic number 7 and is represented by the symbol N. Nitrogen is also known as azote and is a member of the nonmetal group.
nobelium - actinide with element symbol No and atomic number 102.
noble gas - element from Group 8 of the periodic table (e.g., xenon, argon).
noble gas core - shorthand notation used for writing atomic electron configuration in which the previous noble gas configuration is replaced by the element symbol in brackets.
nonbonding electron - electron in an atom that does not participate in a chemical bond with other atoms.
nonelectrolyte - substance that does not dissociate into ions in aqueous solution.
nonmetal - element that does not display metallic properties, typically referring to elements located in the upper right corner of the periodic table.
nonoxidizing acid - an acid that cannot act as an oxidizing agent.
nonpolar bond - chemical bond with even distribution of charge such that it does not have positive or negative poles.
nonpolar molecule - molecule that has even distribution of charge such that it does not have positive and negative sides.
nonspontaneous reaction - chemical reaction that cannot occur without input of external work.
nonvolatile - substance that does not readily evaporate into a gas under ordinary conditions.
normal boiling point - temperature at which a liquid boils at 1 atm of pressure (sea level).
normal concentration - either refers to normal concentration in which the concentration of solutes is the same in two samples or refers to gram equivalent weight of a solute in solution (N).
normality (N) - measure of concentration equal to gram equivalent weight per liter of solution.
normal melting point - temperature at which a solid melts at 1 atm of pressure.
nuclear fission - splitting of atomic nuclei into two or more lighter nuclei, accompanied by an energy release.
nuclear radiation - particles and photons emitted during reactions in the atomic nucleus.
nucleation - process of vapor droplets condensing into a liquid, bubbles forming in a boiling liquid, or particle accretion to grow crystals.
nucleophile - atom or molecule that donates an electron pair to form a covalent bond.
nucleotide - organic molecule comprised of a nucleotide base, ribose or deoxyribose, and one or more phosphate groups.
nucleus - positively charged center of an atom, made from protons and neutrons.
nuclide - an atom or ion characterized by the proton and neutron composition of its nucleus.
null hypothesis - proposition that there is no effect of a treatment or no relationship between an independent and dependent variable.
nutraceutical - a food or part of food that confers health or medical benefits.
O - Octane Number to Oxygen
octane number - value that indicates resistance of motor fuel to engine knock relative to the knock from isooctane (100) and heptane (0).
octet - group of eight valence electrons around an atom.
octet rule - principal that atoms in an atomic bond share their 8 outer electrons.
open system - a system able to freely exchange matter and energy with its surroundings.
orbital - mathematical function that describes the wavelike behavior of an electron.
organic chemistry - study of the chemistry of compounds containing carbon chemical bonded to hydrogen.
osmium - Osmium is the name for the element with atomic number 76 and is represented by the symbol Os. It is a member of the transition metal group.
osmosis - movement of solvent molecules across a semipermeable membrane from a dilute solution to a more concentrated solution, thus diluting it and equalizing concentration on both sides of the membrane.
oxidant - a reactant that oxidizes or removes electrons from another reactant in a redox reaction.
oxidation - loss of electrons by an atom, molecule or ion in a chemical reaction.
oxidation number - the electrical charge of a central atom in a coordination compound if all electron pairs and ligands were removed.
oxidation state - the difference between the number of electrons in an atom in a compound compared with the number of electrons in a neutral atom of the element.
oxide - an ion of oxygen with an oxidation state equal to 2- (e.g., iron oxide).
oxidizer - a reactant that removes electrons from another reactant in a redox reaction.
oxidizing agent - an oxidizer; a reactant that removes electrons from another reactant.
oxyanion - an anion that contains the element oxygen.
oxygen - Oxygen is the name for the element with atomic number 8 and is represented by the symbol O. It is a member of the nonmetal group.
P - Palladium to Pure Substance
palladium - transition metal with element symbol Pd and atomic number 46.
paramagnetism - property of material characterized by being attracted to a magnetic field.
parent atom - atom that undergoes radioactive decay, resulting in one or more daughter atoms.
parent nuclide - nuclide that decays into a specific daughter nuclide during radioactive decay.
partial pressure - the pressure a gas in a mixture of gases would exert if it occupied the volume by itself, at the same temperature.
particulate - small distinct solids suspended in a gas or liquid.
parts per million (PPM) - unit of concentration that is one part solute per one million parts solvent.
Pascal (Pa) - SI unit of pressure equal to the force of 1 Newton per square meter.
Pauli exclusion principle - principle that says no two electrons or other fermions can have identical quantum numbers in the same atom or molecule.
percent composition - percent by mass of each element in a compound.
percent yield - percent ratio of actual yield divided by theoretical yield.
periplanar - describes two atoms or groups of atoms in the same plane as each other with respect to a single bond.
period - horizontal row of the periodic table; elements with the same highest unexcited electron energy level.
periodic law - law that states the properties of elements recur in a predictable and systematic way when they are arranged by increasing atomic number.
periodic table - tabular arrangement of elements by increasing atomic number, ordered according to trends in recurring properties.
periodic trend - regular variation in the properties of elements with increasing atomic number.
periodicity - recurring variations in element properties with increasing atomic number due to trends in atomic structure.
peroxide - a polyatomic anion with molecular formula O 2 2- .
petroleum - crude oil; natural flammable hydrocarbon mixture found in geologic formations.
pH - measure of the hydrogen ion concentration, reflecting how acidic or basic a substance is.
phase - distinct form of matter with uniform chemical and physical properties.
phase change - change in the state of matter of a sample (e.g., liquid to vapor).
phase diagram - chart showing the phase of a substance according to temperature and pressure.
phenolphthalein - an organic pH indicator, C 20 H 14 O 4 .
pH indicator - compound that changes color over a range of pH values.
phlogiston - Phlogiston was believed to be a substance all combustible matter contained and released when burned. Phlogiston theory was an early chemical theory to explain the process of oxidation. Phlogiston had no odor, taste, color or mass. Deflogisticated substances were called the calx of the substance.
pH meter - instrument that measures pH of a solution based on the voltage between two electrodes in the solution.
phosphorescence - luminescence produced when electromagnetic energy (usually UV light) kicks an electron from a lower to higher energy state. A photon is released when the electron falls to a lower state.
phosphorus - nonmetal with element symbol P and atomic number 15.
photon - discrete packet of electromagnetic radiation.
physical change - change that alters the form of matter but not its chemical composition.
physical property - characteristic of matter that may be observed and measured without changing the identity of the sample.
pi bond - covalent bond formed between two neighbor atom unbonded pi orbitals.
pKa - negative base 10 log of the acid dissociation constant; lower pKa correlates to stronger acid
pKb - negative base 10 log of the base dissociation constant; lower pKb correlates with stronger base.
Planck's constant - proportionality constant that relates photon energy to frequency; 6.626 x 10 -34 J·sec.
plasma - state of matter with no defined shape or volume consisting of ions and electrons.
platinum - transition metal with atomic number 78 and element symbol Pt.
plutonium - Plutonium is the name for the element with atomic number 94 and is represented by the symbol Pu. It is a member of the actinide group.
pnictogen - member of the nitrogen element group.
pOH - measure of the hydroxide ion concentration in an aqueous solution.
polar bond - type of covalent bond in which the electrons are unequally shared between the atoms.
polar molecule - molecule containing polar bonds such that the sum of the bond dipole moments is not zero.
polonium - element atomic number 84 with element symbol Po.
polyatomic ion - ion comprised of two or more atoms.
polymer - large molecule made of rings or chains of repeated monomer subunits.
polynuclear aromatic hydrocarbon - hydrocarbon made of fused aromatic rings.
polyprotic acid - acid able to donate more than one hydrogen atom or proton per molecule in an aqueous solution.
positron - the antimatter counterpart to an electron, which has a charge of +1.
potassium - alkali metal with element symbol K and atomic number 19.
potential difference - work required to move an electric charge from one point to another.
potential energy - energy due to an object's position.
PPB - parts per billion
PPM - parts per million
praseodymium - rare earth element with symbol Pr and atomic number 59.
precipitate - to form an insoluble compound by reacting salts or altering a compound's solubility.
precipitation reaction - chemical reaction between two soluble salts in which one product is an insoluble salt.
pressure - measure of force per unit area.
primary standard - very pure reagent.
principal energy level - primary energy signature of an electron, indicated by quantum number n.
principal quantum number - quantum number n that describes the size of an electron orbital.
product - substance formed as a result of a chemical reaction.
promethium - rare earth element with atomic number 61 and element symbol Pm.
proof - volume percentage of ethyl alcohol in an alcoholic beverage.
property - characteristic of matter fixed by its state.
protactinium - actinide with atomic number 91 and element symbol Pa.
proton - component of the atomic nucleus with a defined mass of 1 and charge of +1.
protonation - addition of a proton to an atom, ion, or molecule.
PSI - unit of pressure; pounds per square inch.
pure substance - sample of matter with constant composition and distinct chemical properties.
Q - Quantitative Analysis to Quantum Number
qualitative analysis - determination of the chemical composition of a sample
quantitative analysis - determination of the amount or quantity of components in a sample.
quantum - a discrete packet of matter or energy, plural is quanta
quantum number - value used to describe the energy levels of atoms or molecules. There are four quantum numbers.
R - Radiation to Rutherfordium
radiation - emitted energy in the form of rays, waves, or particles.
radioactivity - spontaneous emission of radiation as particles or photons from a nuclear reaction.
radioactive tracer - radioactive element or compound added to a material to monitor its progress through a system.
radium - Radium is the name for the element with atomic number 88 and is represented by the symbol Ra. It is a member of the alkaline earth metals group.
radon - radioactive gas with element symbol Rn and atomic number 86.
Raoult's Law - relation that states the vapor pressure of a solution depends on the mole fraction of solute added to the solution.
reactant - starting material for a chemical reaction.
reaction - a chemical change that forms new substances.
reaction quotient - Q - ratio of the concentration of products of a reaction to the concentration of the reactants.
reaction rate - the speed at which chemical reactants form products.
reagent - compound or mixture added to a system to produce a reaction or test if one occurs.
real gas - gas that does not behave as an ideal gas because its molecules interact with one another.
redox indicator - compound that changes color at a specific potential difference.
redox reaction - set of chemical reactions involving reduction and oxidation
redox titration - titration of reducing agent by an oxidizing agent or vice versa.
reduction - half reaction in which a chemical species decreases its oxidation number, generally by gaining electrons.
refrigerant - compound that readily absorbs heat and releases it at a higher temperature and pressure.
relative density - ratio of density of a substance to the density of water.
relative error - uncertainty of a measurement compared to the size of the measurement.
relative standard deviation - measure of the precision of data, calculated by dividing standard deviation by the average of data values.
relative uncertainty - relative error; uncertainty of a measurement compared to the size of the measurement.
residue - matter remaining after evaporation or distillation or an undesirable reaction byproduct or a recognizable portion of a larger molecule.
resonance - average of two or more Lewis structures, differing in the position of electrons.
reverse osmosis - filtration method that works by applying pressure on one side of a semipermeable membrane
reversible reactions - chemical reaction in which the products act as reactants for the reverse reaction.
rhenium - transition metal with atomic number 75 and element symbol Re.
rhodium - transition metal with atomic number 45 and element symbol Rh.
RNA - ribonucleic acid, a molecule that codes for amino acid sequences.
roasting - metallurgical process in which a sulfide ore is heated in air to form a free metal or metal oxide.
roentgenium - radioactive element with atomic number 111 and element symbol Rg.
room temperature - temperature that is comfortable for humans, typically around 300 K.
RT - abbreviation for room temperature; ambient temperature that is comfortable for humans.
rubidium - Rubidium is the name for the element with atomic number 37 and is represented by the symbol Rb. It is a member of the alkali metal group.
ruthenium - transition metal with atomic number 45 and element symbol Ru.
rutherfordium - radioactive transition metal with element symbol Rf and atomic number 104.
S - Salt to Synthesis Reaction
salt - ionic compound formed by reacting an acid and a base; sometimes refers only to sodium chloride, NaCl.
salt bridge - connection containing a weak electrolyte located between the oxidation and reduction half cells of a galvanic cell.
samarium - rare earth element with atomic number 62 and element symbol Sm.
saponification - reaction between triglycerides and either sodium hydroxide or potassium hydroxide to form a fatty acid salt called soap and glycerol.
saturated - either a substance in which all atoms are linked by single bonds, a solution that contains maximum dissolved solute concentration, or a thoroughly wetted material.
saturated fat - lipid containing only single C-C bonds.
saturated solution - chemical solution containing the maximum concentration of dissolved solute for that temperature.
scandium - Scandium is the name for the element with atomic number 21 and is represented by the symbol Sc. It is a member of the transition metals group.
science - the systematic study of the nature and behavior of the world using observation and experimentation
scientific law - general rule that explains a body of observations in the form of a mathematical or verbal statement and implies a cause an effect relationship between observations.
scientific method - system of acquiring knowledge and solving problems through observation and experimental testing of hypotheses.
seaborgium - radioactive transition metal with element symbol Sg and atomic number 106.
second quantum number - ℓ, the quantum number associated with the angular momentum of an atomic electron.
selenium - nonmetal with element symbol Se and atomic number 34.
semi-metal - element with a partially filled p orbital, causing it to exhibit properties intermediate between those of metals and nonmetals.
SI - System Internationale, the standard metric system of units.
sigma bond - covalent bonds formed by overlapping of outer orbitals of adjacent atoms.
simplest formula - ratio of elements in a compound.
single displacement reaction - chemical reaction in which an ion of one reactant is exchanged for the corresponding ion of another reactant.
skeletal structure - two-dimensional graphic representation of atoms and bonds in a molecule using element symbols and solid lines for bonds.
sodium - Sodium is the name for the element with atomic number 11 and is represented by the symbol Na.
sol - type of colloid in which solid particles are suspended in a liquid.
solid - state of matter characterized by high degree of organization, with a stable shape and volume.
solidification - phase change that results in formation of a solid.
solubility - maximum amount of solute that may be dissolved in a specified solute.
solubility product - K sp , the equilibrium constant for a chemical reaction in which a solid ionic compound dissolves to yield its ions in solution.
solute - substance that is dissolved in a chemical solution.
solution - homogeneous mixture of two or more substances.
solvent - component of a solution present in the greatest proportion.
specific gravity - ratio of the density of a substance to the density of water.
specific heat - quantity of heat required to raise the temperature of a mass a specified amount.
specific heat capacity - amount of heat needed to raise the temperature of a substance per unit mass.
spectator ion - ion found in the same amount on both the reactant and product sides of a chemical reaction that does not affect equilibrium.
spectroscopy - analysis of the interaction between matter and any portion of the electromagnetic spectrum.
spectrum - characteristic wavelengths of electromagnetic radiation emitted or absorbed by an object or substance.
spin quantum number (Ms) - fourth quantum number, which indicated orientation of intrinsic angular momentum of an electron in an atom.
spontaneous fission - spontaneous splitting of an atomic nucleus into two smaller nuclei and usually neutrons, accompanied by the release of energy.
spontaneous process - process which can occur without any energy input from the surroundings.
standard - reference used to calibrate measurements.
standard hydrogen electrode - SHE, the standard measurement of electrode potential for the thermodynamic scale of redox potentials.
standard oxidation potential - potential in volts generated by an oxidation half-reaction compared to the standard hydrogen electrode at 25 °C, 1 atm pressure and a concentration of 1 M.
standard reduction potential - potential in volts generated by a reduction half-reaction compared to the standard hydrogen electrode at 25 °C, 1 atm pressure and a concentration of 1 M.
standard solution - a solution with a precisely known concentration.
standard temperature and pressure - STP, 273 K (0° Celsius or 32° Fahrenheit) and 1 atm pressure.
state of matter - homogeneous phase of matter (e.g., solid, liquid).
steam distillation - distillation process in which steam or water is added to lower boiling points of compounds.
steel - an alloy of iron that contains carbon.
steric number - number of atoms bonded to a central atom of a molecule plus number of lone electron pairs attached to the central atom.
stock solution - concentrated solution intended to be diluted to a lower concentration for actual use.
stoichiometry - study of quantitative relationships between substances undergoing a physical or chemical change.
STP - standard temperature and pressure; 273 K (0° Celsius or 32° Fahrenheit) and 1 atm pressure.
strong acid - acid that completely dissociates into its ions in aqueous solution.
strong base - base that completely dissociates into its ions in aqueous solution (e.g., NaOH).
strong electrolyte - electrolyte that completely dissociates in aqueous solution.
strontium - alkaline earth with element symbol Sr and atomic number 38.
sublimation - phase transition from solid phase directly to vapor phase.
subshell - subdivision of electron shells separated by electron orbitals (e.g., s, p, d, f).
substrate - medium on which a reaction occurs or reagent that offers a surface for absorption.
substituent - atom or functional group that replaces a hydrogen atom in a hydrocarbon.
substitution reaction - chemical reaction in which a functional group or atom is replaced by another functional group or atom.
sulfur - Sulfur is the name for the element with atomic number 16 and is represented by the symbol S.
supernate - the liquid result of a precipitation reaction.
supersaturated - supercooled; condition in which a liquid has been cooled to a temperature below which crystallization normally occurs, yet without solid formation.
surface tension - physical property equal to the force per unit area needed to expand the surface of a liquid.
surfactant - species that acts as a wetting agent to lower liquid surface tension and increase spreadability.
suspension - heterogeneous mixture of solid particles in a fluid.
synthesis reaction - direct combination reaction; chemical reaction in which two or more species combine to form a more complex product.
T - Tantalum to Tyndall Effect
tantalum - transition metal with element symbol Ta and atomic number 73.
technetium - transition metal with element symbol Tc and atomic number 43.
tellurium - metalloid with element symbol Te and atomic number 52.
temperature - property of matter that is a measure of the kinetic energy of its particles; measure of heat or cold.
terbium - rare earth element with symbol Tb and atomic number 65.
tetrahedral - molecular geometry in which a central atom forms four bonds directed toward the corners of a regular tetrahedron.
Texas carbon - a carbon atom that forms five covalent bonds, forming a structure resembling a star.
thallium - metal with atomic number 81 and element symbol Tl.
theoretical yield - quantity of product that would be obtained if the limiting reactant in a reaction reacted completely.
theory - a well-established explanation of scientific data that can be disproven by a single contrary result.
thermodynamics - scientific study of heat, work, and related properties of mechanical and chemical systems.
thermosetting plastic - a polymer that is made irreversibly rigid upon heating.
thiol - an organic sulfur compound consisting of an alkyl or aryl group and a sulfur-hydrogen group; R-SH.
thiol group - functional group containing a sulfur bound to a hydrogen, -SH.
thorium - Thorium is the name for the element with atomic number 90 and is represented by the symbol Th.
thulium - rare earth element with atomic number 69 with element symbol Tm.
tin - metal with atomic number 50 and element symbol Sn.
tincture - an extract of a sample into a solution, usually with alcohol as the solvent.
titanium - transition metal with element symbol Ti and atomic number 22.
titrant - solution of known concentration used in a titration to determine the concentration of a second solution.
titration - process of adding a known volume and concentration of one solution to another to determine the concentration of the second solution.
torr - unit of pressure equal to 1 mm Hg or 1/760 standard atmospheric pressure.
trans isomer - isomer in which functional groups occur on opposite sides of the double bond.
transition interval - concentration range of chemical species that can be detected using an indicator.
transition metal - element from the B group of the periodic table characterized by having partially filled d electron orbital sublevels.
translational energy - energy of motion through space.
transmute - to change from one form or substance into another.
triple point - temperature and pressure at which the solid, liquid, and vapor phases of a substance coexist at equilibrium with each other.
tungsten - transition metal with atomic number 74 and element symbol W.
Tyndall effect - the scattering of a beam of light as it passes through a colloid.
U - Ultraviolet to Uranium
ultraviolet radiation - ionizing electromagnetic radiation with a wavelength between 100 nm and 400 nm. Sometimes called black light.
UN ID - a four-digit code used to identify dangerous or flammable chemicals. United Nations Identifier
UN number - a UN ID used for the transport of dangerous materials.
unit - a standard used for comparison in measurements.
universal gas constant - usually indicated by R, the gas constant is the Boltzmann constant in units of energy per temperature per mole: R = 8.3145 J/mol·K
universal indicator - a mixture of pH indicators used to measure pH over a wide range of values.
universal solvent - a chemical that dissolves most substances. While water is often called the universal solvent, most nonpolar molecules are insoluble in it.
unsaturated - either refers to a solution that can dissolve more solute or to an organic compound containing double or triple carbon-carbon bonds.
unsaturated fat - a lipid that contains no carbon-carbon double bonds.
unsaturated solution - a solution in which solute concentration is lower than its solubility. All solute present dissolves into the solution.
uranium - element 92 with symbol U.
V - Vacuum to VSEPR
vacuum - a volume containing little to no matter (no pressure).
valence - number of electrons needed to fill the outermost electron shell.
valence bond theory - explanation of bonding between two atoms as a result of the overlap of half-filled atomic orbitals.
valence electron - outer electron most likely to participate in bond formation or a chemical reaction.
Valence Shell Electron Pair Repulsion Theory - molecular model that predicts geometry of atoms in a molecule by minimizing electrostatic forces between valence electrons around a central atom.
vanadium - Vanadium is the name for the element with atomic number 23 and is represented by the symbol V. It is a member of the transition metals group.
Van der Waals forces - weak forces that contribute to intermolecular bonding.
Van der Waals radius - half the distance between two unbonded atoms in a state of electrostatic balance.
vapor - a condensable gas.
vapor pressure - pressure exerted by a vapor in equilibrium with liquid or solid phases of the same substance or the partial pressure of a vapor above its liquid or solid.
vaporization - phase transition from the liquid phase to gas phase.
vector - a geometric object that has both magnitude and direction.
viscosity - how readily a fluid flows, which is the ratio between an applied shear stress and the resulting velocity gradient.
visible light - electromagnetic radiation that can be perceived by the human eye, usually from 380 nm to 750 nm (400 to 700 nm).
volatile - a substance that readily vaporizes.
volume - the three-dimensional space occupied by a solid, liquid, or gas.
volumetric flask - type of chemistry glassware used to prepare solutions of known concentration.
volume-volume percentage - v/v% is the ratio between the volume of a substance in a solution to the total volume of the solution, multiplied by 100%.
VSEPR - see Valence Shell Electron Pair Repulsion Theory
W - Water to Working Solution
water - a compound formed by one oxygen atom and two hydrogen atoms. Usually this refers to the liquid form of the molecule.
water gas - a combustion fuel that contains hydrogen gas and carbon monoxide.
water of crystallization - water the stoichiometrically bound in a crystal.
water of hydration - water stoichiometrically bound in a compound, forming a hydrate.
wavefunction - a function that describes the probability of the quantum state of a particle in terms of spin, time, position, and/or momentum.
wavelength - the distance between identical points of two successive waves.
wave-particle duality - the concept that photons and subatomic particles exhibit properties of both waves and particles.
wax - a lipid consisting of chains of esters or alkanes derived from fatty acids and alcohols.
weak acid - an acid that only partially dissociates into its ions in water.
weak base - a base that only partially dissociates in water.
weak electrolyte - an electrolyte that does not completely dissociate into its ions in water.
wedge-and-dash projection - molecule representation using three types of lines to show three-dimensional structure.
weight - the force on a mass due to the acceleration of gravity (mass multiplied by acceleration).
word equation - a chemical equation expressed in words rather than chemical formulas.
work - force multiplied by distance or the amount of energy needed to move a mass against a force.
working solution - a chemical solution prepared for use in a lab, usually by diluting a stock solution.
X - Xenon to X-Rays
xenon - Xenon is an element with an atomic number of 54 and atomic weight of 131.29. It is an odorless inert gas that is used to fill cathode ray tubes.
x-rays - X-rays are light rays with a wavelength from 0.01 to 1.0 nanometers. Also known as X radiation.
Y - Yield to Yttrium
yield - In chemistry, yield refers to the quantity of a product obtained from a chemical reaction. Chemists refer to experimental yield, actual yield , theoretical yield , and percent yield to differentiate between calculated yield values and those actually obtained from a reaction.
ytterbium - Ytterbium is element number 70 with an element symbol Yb.
yttrium - Yttrium is an element element with an atomic number of 39 and atomic weight of 88.90585. It is a dark gray metal that is used to make alloys for nuclear technology because the element has a high neutron transparency.
Z - Zaitsev Rule to Zwitterion
Zaitsev rule - rule in organic chemistry that states alkene formation from an elimination reaction will produce more highly substituted alkenes.
zeta potential (ζ-potential) - the potential difference across the phase boundary between a liquid and a solid.
zinc - Zinc is the name for the element with atomic number 30 and is represented by the symbol Zn. It is a member of the transition metals group.
zirconium - Zirconium is the name for the element with atomic number 40 and is represented by the symbol Zr. It is a member of the transition metals group.
zwitterion - the dipolar amino acid formed when a hydrogen ion transfers from an acid group to an amine group.
- Fun and Interesting Chemistry Facts
- Stoichiometry Definition in Chemistry
- Examples of Chemical Reactions in Everyday Life
- Chemistry Glassware Types, Names and Uses
- List of Radioactive Elements and Their Most Stable Isotopes
- Element Symbol Definition in Chemistry
- Molarity Definition in Chemistry
- What Is an Element in Chemistry? Definition and Examples
- Oxidation Definition and Example in Chemistry
- Printable Periodic Table Image and Periodic Table Wallpaper
- Periodic Table of the Elements With Accepted Atomic Masses
- How to Calculate Atomic Mass
- The Difference Between Intensive and Extensive Properties
- The Difference Between Homogeneous and Heterogeneous Mixtures
- A List of All the Elements of the Periodic Table
- Valency of Elements on the Periodic Table

IMAGES
COMMENTS
This glossary of chemistry terms is a list of terms and definitions relevant to chemistry, including chemical laws, diagrams and formulae, laboratory tools, glassware, and equipment.Chemistry is a physical science concerned with the composition, structure, and properties of matter, as well as the changes it undergoes during chemical reactions; it features an extensive vocabulary and a ...
analytical chemistry - Analytical chemistry is the chemistry discipline concerned with the chemical composition of materials. Analytical chemistry also is concerned with developing the tools used to examine chemical compositions. angstrom - An angstrom is a unit of measurement for very small distances. The symbol for angstrom is Å.
This is a list of important chemistry vocabulary terms and their definitions. Look up words in this online dictionary. This is a list of important chemistry vocabulary terms and their definitions. ... Projects & Experiments Scientific Method Biochemistry Physical Chemistry Medical Chemistry Chemistry In Everyday Life Famous Chemists Activities ...
An experiment includes many scientific method vocabulary terms. The scientific method involves a hypothesis, variables, controls, experiments, and other concepts and terms that may be confusing. This is a glossary of key scientific method vocabulary terms and their definitions. Glossary of Scientific Method Words
The chemistry dictionary offers definitions and examples of important chemistry and chemical-related terms, which are listed in alphabetical order. For each term, a brief definition is given. Each link leads to a more comprehensive discussion of the word. Additional definitions are also available. Chemistry Terms Starting with A to Z
Here is a glossary of important science experiment terms and definitions: Central Limit Theorem: States that with a large enough sample, the sample mean will be normally distributed. A normally distributed sample mean is necessary to apply the t- test, so if you are planning to perform a statistical analysis of experimental data, it's important ...
Our website is a free online chemistry dictionary containing over 1,800 chemistry terms and definitions. We invite Everyone to use our dictionary of chemistry terminology to help further their knowledge, students and teachers alike. To start browsing the dictionary, choose a letter to list all the terms starting with that letter. ...
The nitrogen triiodide chemistry demonstration is a remarkable and attention-grabbing experiment that students should try under the guidance of a knowledgeable instructor. By reacting iodine crystals with concentrated ammonia, students can precipitate nitrogen triiodide (NI3), a highly sensitive compound.
The Celsius scale is a common temperature scale in chemistry. Indeed / Getty Images. cadmium - Cadmium is the name for the element with atomic number 48 and is represented by the symbol Cd. It is a member of the transition metals group. caffeine - Caffeine is a chemical substance naturally found in tea and coffee and added to colas.. calcium - Calcium is the name for the element with atomic ...
Chemistry experiment terms. Flashcards. Learn. Test. Match. Flashcards. Learn. Test. Match. Created by. jaydon_biggs. Terms in this set (20) Categoric variable. Have labels not quantities e.g types of metals. Reproducible. Experiment done again in a different way or by someone else and get the same result ...